Organics in the boiler and steam: Good or bad?
Organic compounds pioneered boiler water chemical treatment when lignosulfonates derived from oak bark were used to minimize calcium carbonate scaling. Today’s organic boiler treatment methods include the addition of neutralizing amines and oxygen scavengers to feedwater and polymers to boiler water. The literature is rich with documentation of the methods’ mechanisms and results as well as the products into which treatment chemicals break down as they are exposed to the temperatures and pressures of the steam cycle.
Both sides debating whether organics do more harm than good to boilers can trot out data to support their position. But history is on the side of the proponents. For years, the vast majority of fossil-fueled and nuclear plants have treated their boiler water with carbon-based chemicals—amines, for example. The positive effect of these chemicals on pH and corrosion prevention are well known. There have been no cases of turbine corrosion that can be directly and unequivocally tied to organic chemical treatment. However, there may have been some instances of corrosion in the turbine and condenser due to the presence of other, larger organic molecules, such as those coming in with makeup water.
Based on experience, utility chemists are primarily concerned about stress-corrosion cracking, corrosion fatigue, and flow-accelerated corrosion in the steam turbine, particularly its low-pressure section (Figures 1 and 2). Corrosion starts where condensation begins, in the phase transition zone, where saturated and superheated steam coexist. The interaction of wet and dry environments in this area enables any steam contaminant to rise in concentration to levels that may be corrosive to materials of turbine blades and rims. Among the chemicals whose corrosion mechanisms have been studied extensively are chlorides and sulfates. But less known is whether carboxylic acids—such as formic and acetic acid—or carbon dioxide (CO2) are potentially as destructive as chlorides and sulfates, and at what concentrations.

1. Weakest link. The final stages of the low-pressure section of a steam turbine are the most vulnerable to corrosion. Courtesy: David Daniels

2. Reasonable doubt. Though organic compounds have never been proven as a cause of stress-corrosion cracking, they’re one of the concerns raised by those who want to limit organic compounds in steam. Courtesy: David Daniels
Where organics come from
There are two primary sources of carbon-based molecules in a boiler or in the steam it produces: compounds that enter with makeup water and organic treatment chemicals.
The natural organic matter (NOM) that comes in with makeup water is quite diverse. Constituents include large, complex, naturally occurring organic molecules such as humic acid (from plant degradation) and polysaccharides (generated by bacteria). `The presence and concentrations of these compounds depend largely on the makeup water source and the time of year.
Many of these compounds can cause problems at the front end of the water treatment plant, fouling reverse osmosis (RO) membranes and ion exchange resins. Any compounds not removed by the treatment plant pass through to the makeup water, where they break down into organic acids and CO2, elevating cation conductivity in the process. Many plants have found during precommissioning that keeping cation conductivity below the limits recommended by the turbine manufacturer would require expensive modifications to makeup pretreatment to address NOM.
If potable water or secondary wastewater effluent is used for makeup, organic compounds in that water may be chlorinated. Besides forming trihalomethanes, chlorine can attach itself to polysaccharides, giving it a free ride through the pretreatment system. In one autoclave test, feedwater-quality water was heated for seven days at 572F. At the end of the test, the chloride content was about 10 times higher than at the beginning. The best explanation for the rise in concentration: Chloride went undetected in the original sample because it was tightly bound to an organic compound.
Until recently, it has been difficult to identify and classify carbon-based organic compounds. Traditional total organic carbon analyzers convert all carbon species into CO2 and measure the amount generated—an indiscriminate technique. A new technique developed in Germany, liquid chromatography–organic carbon detection (LC-OCD), has the potential to differentiate these compounds and improve the determination of appropriate treatment technologies.
Figure 3 shows the results of a LC-OCD analysis before and after a cation exchanger. The water source was a municipal supply from which chlorine was removed. Here, the humic acids fouled the resins and shortened run times.

3. Looking for organics. Organic fouling of a cation resin can be detected by a new application of liquid chromatography. Courtesy: Stefan Hufer
Where organics end up
When organic compounds enter the boiler they are exposed to extreme temperature and pressure. If the compound is volatile, it is carried over into the superheater and reheater tubing. Under these conditions, most organic compounds break down. Amines and nitrogen-containing oxygen scavengers produce ammonia, and carbon molecules form CO2 and a host of carboxylic acids such as formic, acetic, butyric, propionic, and glycolic acid. Chlorinated organic compounds can produce hydrochloric acid vapors, and organic sulfonates can form sulfuric acid.
By and large, these breakdown products are more volatile in their acid form than as sodium salts. For this reason, the presence of sodium salts in the boiler may favor phosphate and caustic treatment regimes over an all-volatile treatment.
The utility industry has recently expanded its knowledge concerning the effect of organic acids and other breakdown products on low-pressure turbine blades. Of all the organic breakdown products, it turns out that CO2 is the most innocuous. Research indicates that, even at relatively high concentrations, CO2 at 212F will not depress the at-temperature pH below that of pure water. However, at cooler temperatures, CO2 can lower pH and raise iron corrosion rates in air- and water-cooled condensers—but not in turbines. An amine can counter this effect by raising the pH and reducing the solubility of iron.
By comparison, carboxylic acids such as formic and acetic acid in first condensate depress pH, but only slightly. Some research suggests that acetate has the ability to complex, or chemically tie-up, iron corrosion products. Complexing agents help maintain iron in solution, and that may further (or accelerate) mechanisms such as stress-corrosion cracking.
Chloride and sulfate have a significant and well-documented depressing effect on the pH of first condensate. They thus cause, or at least facilitate, corrosion fatigue and stress-corrosion cracking. For this reason, the most significant risk to turbines from organics may come from chloride-containing organic compounds created by the chlorination of organics in makeup not removed by a demineralizer plant. Polisher resins have also been found to be a source of sulfonated organic compounds. This has been an issue for chemists at pressurized-water reactors (PWRs), who closely monitor sulfate levels.
As steam condenses in the turbine, condensate finds its way to the stationary blades and then typically is drained away. Designers place the drains where they anticipate that condensate will collect during steady-state full-load operation. But in turbines that are cycled frequently, the areas in which condensate collects may not coincide with the drain slots. In such cases, the result has been flow-accelerated corrosion (FAC) of the area around the steam seals. The degree to which FAC is facilitated by the presence of organic acids is unclear. In many cases, the problem can be solved simply, by upgrading the material in this area to a more corrosion-resistant alloy.
Membranes or Mother Nature?
Membrane technologies are well suited to removing NOM—particularly high-molecular-weight molecules such as humic acid—from water sources. Ultrafiltration membranes have proven capable of removing 25% to 40% of NOM. Using a different mechanism, reverse osmosis can remove between 65% and 85%. Combining the two techniques can achieve an impressive 75% to 96% removal rate, with larger organic molecules more easily removed than smaller ones.
Figure 4 illustrates how using membranes and RO together in a demin water plant can dramatically reduce total organic carbon as well as the concentration of several organics. Organics removal is another good reason to consider using both ultrafiltration and RO to treat your water.
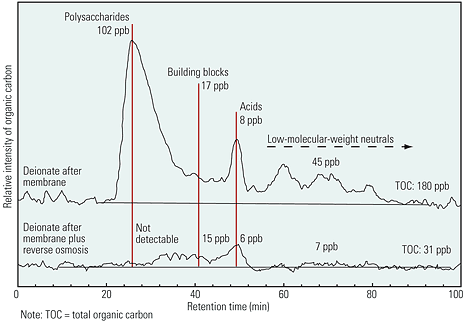
4. Use your ‘brane. Using membranes and reverse osmosis can dramatically reduce concentrations of organics. Courtesy: Stefan Hufer
Traditionally, carbon filters are used upstream of an ion-exchange train to remove chlorine and some organic compounds. But they turn out also to be an effective way to remove NOM via another mechanism. Polysaccharides will initially stick to the activated carbon of a new bed. Once the concentration builds up, bacteria will flourish and begin to metabolize incoming organic compounds. If allowed to continue to develop on the carbon, this layer of bacteria creates a bio-reactor of sorts. NOM reduction across such a biologically active carbon filter can be significant. One author calls it the "cheapest and most reliable technique" for removing polysaccharides from boiler feedwater.
Neutralizing amines
Neutralizing amines have been used extensively by operators of utility and industrial boilers for many years. The first application of amines to feedwater treatment was in the late 1940s. In the 1980s, they were in common use in many boilers. The nuclear power industry, which had been using morpholine or ammonia in the secondary water of PWRs, made a wholesale switch in the 1990s to "advanced amines" such as ethanolamine, dimethylamine, and 3-methoxypropylamine. By 2005, all but one of 55 PWRs surveyed were using one or more amines (including morpholine) to help reduce iron levels in their secondary water.
Amines have two major advantages over ammonia. One is their higher basicity, which produces a greater rise in the pH of condensate per ppm of compound (Figure 5). The other is their higher relative volatility (RV), defined as the ratio of the amount of the substance (ammonia or amine) in condensate vapor to its amount in liquid-phase water at a given temperature and pressure. The higher the RV, the higher the percentage of the compound in the vapor phase. The lower the RV, the more quickly the amine falls out of solution and back into the condensate. As condensate forms at higher temperatures, a high-volatility amine stays in the vapor.
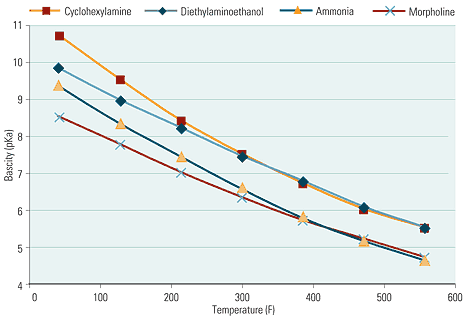
5. Basic instinct. Because most amines have a higher basicity than ammonia, they produce a greater increase in pH of condensate. Source: POWER magazine
Amines of different volatilities have different applications (Figure 6). Straight steam turbine–based power plants call for the use a low-volatility amine such as morpholine. By contrast, cogeneration plants and plants that produce process steam for export need an amine such as cyclohexylamine whose high volatility enables it to travel all the way to the end of the line to elevate pH and thus minimize corrosion around steam traps and in condensate return sets. Often, a blend of several amines of different volatilities is used to cover all the bases.
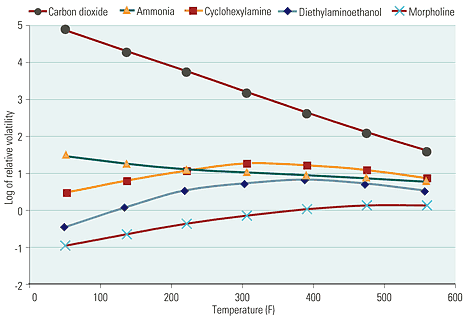
6. Different strokes. This graph shows the relative volatility of several common neutralizing amines as a function of temperature. The choice of amine depends on the application. Source: POWER magazine
One apparent advantage that amines have over other organic treatments is that, when they break down, they produce ammonia as well as acids. Ammonia has the opposite effect of any organic acid: It increases the pH of condensate. Though that is reassuring at the sample panel, in reality, the rise in pH does very little for the first condensate in the turbine. The volatility of ammonia keeps it in vapor form until it reaches the condenser. That said, the effect of ammonia condensation on the pH of the condensate in an air- or water-cooled condenser can be significant.
The pluses and minuses of amine use are the focus of a multiyear study by the American Society of Mechanical Engineers’ Research & Technology Committee on Water and Steam in Thermal Power Systems (see box).
Oxygen scavengers
Plants with copper-alloy feedwater heaters must use oxygen scavengers or reducing agents to achieve a passive layer of cuprous oxide on their copper tubing. Here again, there are a variety of chemical options ranging from eschewing carbon to using large organic molecules.
Hydrazine is the scavenger of choice for power plants, particularly those sensitive to organic compounds or their byproducts in steam and condensate. The molecule contains no carbon and is an aggressive scavenger and passivator of iron oxide. For years, carbohydrazide (which contains only one carbon atom per molecule) was marketed by Nalco Chemical Co. as ElimiNOx; it is now available as a generic. At feedwater temperatures, one molecule of carbohydrazide forms two hydrazine molecules and one molecule of CO2. As mentioned earlier, CO2 does not affect the pH of first condensate in the turbine, although it does increase the cation conductivity of feedwater.
Other volatile oxygen scavengers in use are larger organic molecules such as DEHA (N,N,-diethylhydroxylamine) and methyl-ethyl ketoxime (both with 4 carbon atoms per molecule), and hydroquinone and erythorbate (d-isoascorbic acid), both weighing in at 6 carbons per molecule. The amount of these chemicals needed to achieve the desired oxygen reduction and copper passivity varies by chemical and application. What all the carbon-based scavengers have in common, however, is that they break down (either by reacting with iron oxide or under heat and pressure) to smaller organic molecules and CO2. Obviously, the more of these chemicals you add to your steam cycle and the more carbon they have in them, the higher the concentrations of by-products in the steam and feedwater will be. If there are any corrosion ramifications of the presence of organic acids in the steam, the plant with the highest concentrations will be the first to see them. This is one of the reasons that many plants have gone back to hydrazine or at least carbohydrazide as their oxygen scavenger of choice and try to add only what is needed to keep the copper passivated.
Polymerized dispersants
Dispersants were among the first chemicals used to treat boiler water. Modern, acrylate-based boiler treatment polymers now have been available for more than 20 years. These long-chain organic molecules are still primarily used by industrial boilers and boilers that operate at pressures lower than those of the typical utility boiler. In an industrial plant, it may be very difficult to keep the amount of iron corrosion product that returns with the condensate to a desirable level. Such cases may call for a dispersant.
At stand-alone power plants, it is difficult to justify the use of dispersants. Their purpose is to disperse corrosion products and contamination long enough to be carried out with boiler blowdown. However, at plants that treat their makeup supply with RO/mixed-bed ion exchange or continuous deionization, the makeup water supply is consistently excellent. In these cases, the primary source of potential contamination is the condenser. If the condenser is tight, there is very little contamination entering the boiler and therefore little reason to open the blowdown. Without significant blowdown, the dispersant ends up circulating in the boiler for a long time. It is important to remember that the use of a dispersant also requires the use of the continuous blowdown to remove dispersed iron. Accordingly, the use of any high-molecular-weight organic molecule, such as a dispersant, in a system with high-quality condensate should be considered carefully.