Use predictive techniques to guide your mercury compliance strategy
Under the Clean Air Mercury Rule (CAMR) issued by the U.S. Environmental Protection Agency in March 2005, the estimated 48 tons of mercury (Hg) emitted annually by coal-fired power stations nationwide must fall to 34 tons by 2010 and to 15 tons by 2018. A handful of states have already mandated shorter time frames, deeper reductions—or both—for their Hg emissions.
The U.S. Department of Energy’s National Energy Technology Laboratory, in collaboration with EPRI and several prominent utilities, has been conducting a comprehensive field-testing program to evaluate available Hg control options. Test data from more than 40 full-scale flue gas cleaning systems for an assortment of Hg control technologies are already available at www.netl.doe.gov/technologies/coalpower/ewr/mercury/index.html.
The program also has tested the so-called "inherent Hg removal" of particulate collection devices (PCDs) and scrubbers. Finally, most utility companies in Midwest, North Central, Mid-Atlantic, and Southeast states have recorded their own internal Hg test data. All these data can help utilities assess the performance and costs of available Hg control options and their compatibility with various coals and gas cleaning system configurations.
With so much data available, we might even expect someone to have developed engineering models that can predict the Hg emissions from a particular plant burning coal with specific properties accurately enough for compliance planning. Certainly, many statistical methods have been used to sort out the underlying factors that affect Hg emissions. But none has yet been able to deliver the level of quantitative accuracy that utility executives need to ensure regulatory compliance. Reason: A bewildering array of factors determines whether Hg will be collected on flyash, sorbents, or an SO2 scrubber’s slurry, or whether it will exit the stack.
Mercury species
To manage Hg emissions, one needs to know the chemical form or "speciation" of Hg as it enters various air pollution control devices in a flue gas cleaning system. Mercury is present in flue gas in three forms:
- Elemental (Hg0) vapor in the flue gas
- Oxidized components (Hg2+) in the flue gas
- Attached to unburned carbon (UBC) or other components of flyash (Hg-P)
The speciation is critical because although Hg2+ is highly water-soluble and therefore easily retained in wet scrubber solutions, Hg0 is not soluble at all. Both Hg0 and Hg2+ vapors may become attached to flyash or sorbents to form Hg-P, and any kind of PCD collects essentially all the Hg-P. The problem is that the proportions of Hg0, Hg2+, and Hg-P often are very different for different fuels burned by plants with the same downstream cleanup configuration, and just as different for the same fuel at plants with different cleaning systems.
There are many instances in the testing literature of variations in Hg speciation in what appeared to be the same fuel in the same cleaning configuration on different days in the same test campaign. Since these variations have confounded statistical attempts to predict Hg emissions, they are worth exploring in greater detail.
Path of least resistance
The clearest perspective on Hg removal comes from recognizing Hg speciation as a marker for the various chemical reactions that transform mercury as it passes through a gas cleaning system (Figure 1). The starting point for this chemistry is very well defined because all the Hg fed into the furnace with the fuel will be present as Hg0 at the furnace exit. But after the flue gas is cooled by an economizer, the Hg0 starts to oxidize into Hg2+ via reactions in the flue gas, and does so at an even faster rate via reactions on suspended particles of UBC.
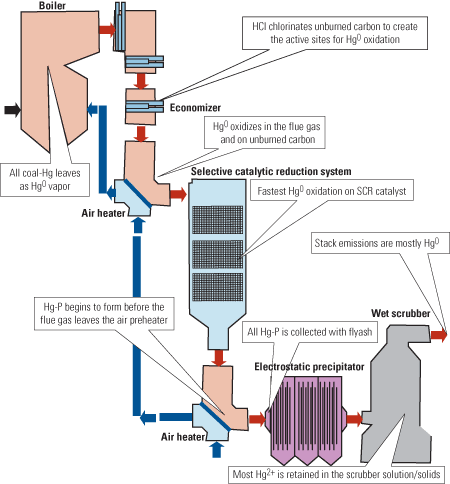
1. Mercury lifecycle. Hg speciation changes from pure Hg0 vapor at the furnace exit to changing mixtures of Hg0, Hg2+, and Hg-P, depending on the levels of Cl and unburned carbon, whether an SCR system is present, and many other cleaning conditions. Source: Niksa Energy Associates LLC
Hg0 oxidizes even faster along the catalyst in a selective catalytic reduction (SCR) system. By the time the flue gas leaves the air preheater, it is cool enough for Hg0 and Hg2+ to bind to UBC or to any sorbents injected to recover it as Hg-P in a downstream PCD. The mixture of Hg0 and Hg2+ leaving the PCD will be stripped of almost all its Hg2+ by a wet scrubber. Therefore, most of the Hg that exits the stack will be Hg0 vapor whenever a scrubber is present.
Hg chemistry is governed by changes in temperature through the cleaning system, the residence time of its various devices, and the concentrations of the participating species. But none of these parameters is the same for different gas cleaning systems, or even for the same system on different days. As a result, the otherwise confounding variations in Hg speciation measurements are due mostly to differences in the Hg chemical environment that naturally arise among utility gas cleaning systems.
All plants are unique
Among a coal’s properties, its chlorine (Cl) content and loss-on-ignition (LOI) are most critical to mercury reduction. That’s because chlorine is the species that actually oxidizes Hg0, and because the UBC component of LOI both accelerates Hg oxidation and acts as the most effective inherent Hg sorbent in flyash. Most bituminous coals contain much more chlorine and generate much more LOI than low-rank coals, and the expected difficulties of oxidizing the Hg from low-rank coals are well established in the test data.
But something even more important lies beyond that generic trend. A coal’s Cl content is a random variable that does not correlate with any other coal property. Chlorine content can routinely vary by a factor of two or three during the course of Hg field testing over several days, and in one recent 30-day test program it changed by a factor of five. These variations in coal-Cl change the Hg chemistry and are partly responsible for the variations in Hg speciation data.
Compounding the problems caused by variability in coal properties, the conditions of flue gas cleanup also directly affect the underlying Hg chemistry and therefore directly determine Hg emissions rates (Table 1). As examples, different flue gas quench rates imposed by different designs of economizers and air preheaters can affect Hg reaction rates. Longer ductwork sustains more chemistry than shorter connections. Utility SCR systems are operated at space velocities from 1,700 to 5,000 hr-1, which changes the residence time available for catalytic Hg oxidation by almost a factor of three. In addition, different SCR units use different flow passages and active ingredients that also affect Hg oxidation rates.
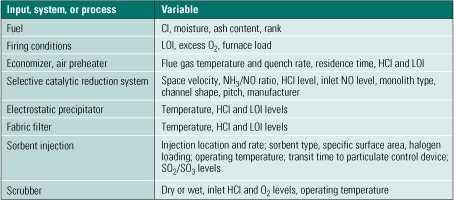
Table 1. Flue gas cleaning conditions that affect Hg chemistry. Source: Niksa Energy Associates LLC
The operating temperature of PCDs is crucial to their efficacy, which is why hot-side electrostatic precipitators (ESPs) collect much less Hg than do cold-side ESPs. The excellent contact between flue gas and flyash in the filter cakes of fabric filters enhances Hg0 oxidation and utilizes the full capacities of UBC or sorbent for Hg capture. Spray dryer-absorbers usually perform as well as or better than wet scrubbers, but the details of a scrubber’s design have little bearing on its Hg retention.
These aspects of Hg chemistry help to explain much of the most erratic content in the field testing database. But they do little to help utility planners decide which Hg control strategies would work best at their plants. Utilities that aggressively manage fuel quality by burning and blending subbituminous and bituminous coals from different regions will need to contend with the full spectrum of Hg reaction conditions. But even those utilities that limit their fuels to a certain type of coal from a specific region will still need to manage the impact of gas cleaning conditions, especially if their plants use or plan to use SCR systems and scrubbers. In practice, it is impossible for most utilities to conduct enough Hg field tests to cover the ranges of coal quality, cleaning conditions, and cleaning system configurations that will have to be managed through the second stage of CAMR implementation.
Better mousetrap
What is needed is the means to generalize the results of a few tests to the operational multitude of Hg conversion conditions—in other words, a way to accurately forecast how changing fuels will affect Hg emissions, whether the Hg removal effectiveness of existing and planned gas cleaning systems will be enough to meet pending regulations, and where to add external Hg controls to get the biggest bang for the buck. Models are normally used to satisfy these imperatives, but statistical models have so far failed to extrapolate from their correlation databases to new situations. That’s because statistical approaches work best in static situations but are poorly suited for dynamic environments like the ones that determine Hg emissions.
The modeling tools developed by Niksa Energy Associates solve that problem. Because they are based on dynamic reaction analysis (DRA), their equations account for the rates of all chemical reactions in a gas cleaning system that affect Hg emissions (Figure 2). As the flue gas cools along a gas cleaning system, the reaction rates in a DRA-based model automatically slow down at different rates, and high-temperature bottlenecks give way to new bottlenecks at cooler temperatures. Different fuel qualities and cleaning conditions also affect these rates, as does the injection of chlorine or other halogens and sorbents into the flue gas stream. The rates used for catalytic Hg oxidation along SCR systems are faster than the rates for the in-flight Hg chemistry.

2. Predicting Hg levels. Users enter fuel properties and gas cleaning conditions into simulations, based on dynamic reaction analysis, of Hg speciation along the flue gas cleaning system. Once the predictions have been validated with baseline Hg test data, the simulations can predict how emissions will vary as fuels, cleaning conditions, and the configuration of pollution control systems change. Source: Niksa Energy Associates LLC
Once the essential chemical information has been introduced into the rate equations, they can be solved to predict the complete Hg speciation at all points along a gas cleaning system (Figure 3). In a system with an SCR/ESP/scrubber combination, Hg0 begins to oxidize as it moves out of the economizer into the SCR system. The oxidation rate accelerates along the SCR catalyst and then slows down as the flue gas moves through the air preheater. Hg-P begins to form at the back end of the air preheater and reaches a level determined by the LOI level. At the ESP outlet, the high level of Hg2+ paves the way for substantial Hg retention in the scrubber.

3. Predicted Hg speciation from the furnace exit through the electrostatic precipitator. In a plant with an SCR system (left), Hg0 begins to oxidize upstream of the SCR inlet and will be converted much faster along the SCR catalyst, forming Hg-P downstream of the air preheater inlet. In a plant without an SCR system (right), the Hg0 oxidation rate upstream of the air preheater is much slower, but activated carbon injected upstream of the precipitator collects most of the Hg0 and Hg2+. Source: Niksa Energy Associates LLC
At plants without an SCR system, the Hg2+ level upstream of the ESP will be lower for similar levels of coal-Cl and LOI. But activated carbon injection upstream of the ESP can still collect most of the Hg as Hg-P in the ESP flyash.
If a scrubber is downstream of the PCD, the predicted Hg speciation at the PCD’s outlet is used as the input for an equilibrium analysis of Hg retention in a wet scrubber or spray dryer-absorber. Because this analysis can accurately predict even test-to-test variations in Hg2+ retention by wet scrubbers (Figure 4), it is not necessary to specify the chemical reaction rates in scrubber solutions. However, the analysis does not predict Hg0 re-emission from dissolved Hg2+, although this contribution rarely exceeds the measurement uncertainties for the vast majority of scrubbers that have already been tested for Hg retention.
4. Achieving equilibrium. An equilibrium analysis of Hg2+ retention in commercial wet scrubbers accurately describes the wide range of Hg scrubbing efficiencies recorded at different sites and even on different days in the same test campaign. Source: Niksa Energy Associates LLC
DRA is usually the province of chemists and chemical engineers working in the petrochemical, specialty chemicals, automotive, and electronics industries. Utilities developed an appreciation of the value of mathematical models based on DRA during the development of in-furnace NOx abatement strategies and in the management of fuel quality impacts on furnace operations.
EPRI took a major step forward in developing DRA into a predictive capability for Hg emissions in early 2005 when it authorized a program to validate and evaluate the predictive capabilities of two DRA-based models, as a way of overcoming the shortcomings of statistical models. The ProMerc model from Reaction Engineering International Inc. (www.reaction-eng.com) combines DRA with statistical models for some air pollution control devices. Niksa Energy Associates’ MercuRator model applies DRA up to the scrubber inlet and then uses a slightly simpler approach to predict Hg2+ retention in the scrubber. The remainder of this article uses predictions from MercuRator to illustrate the performance and benefits of this modeling approach.
Validating the model
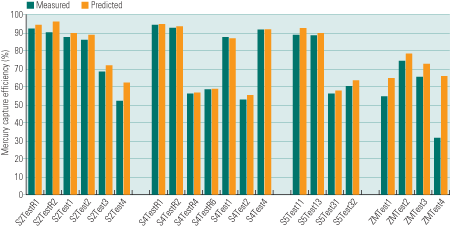
Test data from more than 50 full-scale gas cleaning systems have already been interpreted by MercuRator. Each individual test was simulated independently with readily available test-specific coal properties and gas cleaning conditions. The predicted Hg emissions rates are directly compared with test data (Figure 5) for systems with PCDs alone and SCR/PCD/scrubber combinations, and for systems that use sorbent injection for Hg control. The measurement uncertainties usually fall within 10% to 15% of the total Hg inventory. The standard deviation (SD) of the predictions (Table 2) are well within this tolerance for SCR/PCD and SCR/PCD/scrubber combinations, very close to it for sorbent injection applications and systems with PCDs only, and somewhat greater for PCD/scrubber combinations.
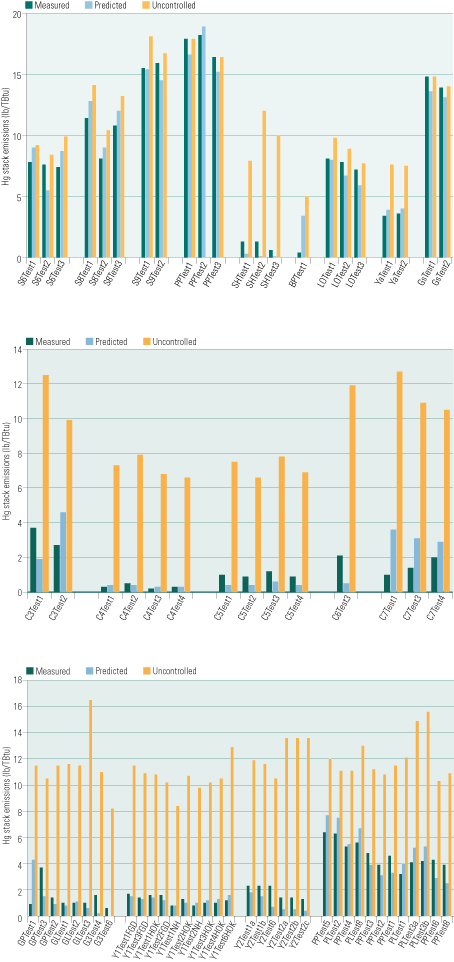
5. Educated guesses. A comparison of measured (green bars) vs. predicted (blue bars) Hg emission rates for flue gas cleaning systems with particulate control devices only (top), SCR/ESP/scrubber combinations (middle), and activated carbon injection (bottom). Uncontrolled emission rates also are shown, as orange bars. The rates were predicted for individual tests to make test-to-test variations apparent. Source: Niksa Energy Associates LLC

Table 2. MercuRator prediction variance. Source: Niksa Energy Associates LLC
Since its predictions have proven accurate for the most popular gas cleaning configurations, MercuRator now is being used by several utilities to support their Hg compliance activities. Researchers at Tennessee Valley Authority (TVA), for example, wanted to predict the impact on Hg reduction of adding SCRs to the utility’s large baseload plants that have been or are being equipped with wet scrubbers. They also wanted to assess any changes in the inherent Hg control when the SCR system is taken out of service.
At TVA, baseline data from an SCR/ESP/scrubber combination with and without the SCR system in service were used to ensure that the predictions were accurate for the coals and cleaning conditions of interest. Then, a library of MercuRator simulations compiled the Hg removal rates for broad ranges of coal-Cl, LOI, furnace load, and SCR conditions (including SCR bypasses).
The estimated impact of variations in coal chlorine content (Figure 6) is qualitatively different with and without an SCR system in service. When the system is on-line, the impact of chlorine becomes stronger when the variable rises above 0.05% by weight, but then it weakens considerably as the level passes 0.12%. Since the TVA baseline levels were 0.154%, excursions into the Cl-sensitive operating regime will probably be infrequent if a supply of similar coal is secured. This insensitivity arises because the SCR simulated is overdesigned for Hg0 oxidation. By contrast, when the SCR system is off-line, Hg removal increases in direct proportion to coal chlorine content over its full range.
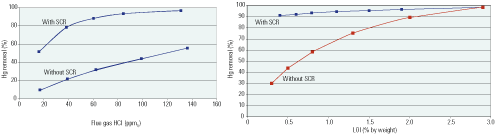
6. Off-line and off the hook. Variations in a coal’s chlorine content and LOI have a much greater impact on Hg removal effectiveness when the plant’s SCR system is out of service. Source: Niksa Energy Associates LLC
At TVA, there were also markedly different impacts of LOI variations with and without the SCR system in service. When the system was on-line, LOI variations were negligible due to compensation by Hg0 oxidation across the SCR system. For example, the Hg2+ levels at the SCR inlet increased from 39% to 93% when LOI was increased from a negligible level to 3%. That’s because the higher LOI accelerated the in-flight Hg0 oxidation on UBC. However, the levels at the SCR outlet increased by less than 10% over the same range of LOI. When the SCR system was off-line, the Hg removal effectiveness increased from 30% to 99% as LOI increased to 3%, matching the performance of the baseline system with SCR at 3% LOI. (Don’t expect the numeric values in this paragraph to apply to other gas cleaning conditions.)
Other companies are using MercuRator predictions to identify the optimal injection rate of activated carbon. When the carbon is injected upstream of a cold-side ESP, the optimal rate is elusive because it depends on the inherent levels of chlorine and LOI in the system, as well as on the temperature and sorbent transit time available for Hg capture.
For low chlorine levels, Hg removal is limited by the total pool of chlorinated sites that can form on the injected carbon (Figure 7). As a result, adding more carbon redistributes the chlorine over a larger population of carbon particles, but it does not expand the pool of active sites for Hg removal. Conversely, when chlorine is plentiful, the performance of the sorbent is enhanced by Hg absorption on suspended UBC in flyash upstream of the sorbent injection point. In other words, carbon sorbents perform better at higher LOI levels, provided that sufficient chlorine is present to use all the active sites on the UBC and the carbon sorbent.
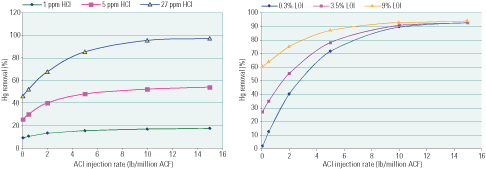
7. Impact on ACI of chlorine and LOI. These graphs show the Hg removal effectiveness of activated carbon injection at various coal-Cl levels for a fixed LOI (left), and at various LOI levels for a fixed coal-Cl content (right). Additional LOI enhances sorbent performance, provided that sufficient Cl is available. Source: Niksa Energy Associates LLC
The information from sensitivity studies like these is impossible to obtain from field testing because all of the important variables can never be regulated in a full-scale cleaning system. And, perhaps more importantly, DRA-based simulations can be run for a fraction of the costs of testing.
—Dr. Stephen Niksa ([email protected]) is president and David P. Bour ([email protected]) is marketing consultant for Niksa Energy Associates LLC. Thomas A. Burnett ([email protected]) is senior specialist and Dr. Naresh B. Handagama PE ([email protected]) is an engineering specialist for Tennessee Valley Authority.