Why Sulfur Oxides Are Bad and How Flue Gas Desulfurization Technology Works
Sulfur oxides (SOx) have several harmful effects both to the environment, and to human and animal health. Much of the SOx in the atmosphere comes from the burning of fossil fuels by power plants and other industrial facilities. However, the use of flue gas desulfurization technology cuts SOx and particulate matter emissions substantially.
Sulfur oxides (SOx) are a group of molecules made of sulfur and oxygen atoms, such as sulfur dioxide (SO2) and sulfur trioxide (SO3). SOx are pollutants that contribute to the formation of acid rain, as well as particulate pollution.
Some SOx are gases, and some are liquids or solid particles. SO2 is the most dangerous of the SOx molecules. It is a colorless gas that smells like burnt matches. SO3, on the other hand, is often a colorless or white solid that creates white fumes in the air and has strong reactions with water. Both SO2 and SO3 react to form sulfuric acid, which is toxic to living tissue and is the main component of acid rain.
While some natural sources, such as volcanoes, contribute to SOx in the atmosphere, the vast majority of SO2 released comes from burning fossil fuels to generate electricity and heat. When coal and oil burn, the sulfur in them combines with oxygen in the air to produce SOx. Natural gas–fired units also produce SO2, but far less. The U.S. Government Accountability Office found that coal-fired units produced more than 90 times as much SO2 on a per-MWh-generated basis compared to natural gas–fired units, due mainly to the higher sulfur content in coal.
SO2 emitted into the atmosphere can undergo chemical reactions to form compounds that can travel long distances. These chemical compounds take the form of tiny solid particles or liquid droplets and can remain in the air for days or even years, according to the U.S. Environmental Protection Agency (EPA).
Health and Environmental Effects of SO2
At some point, however, the pollutants return to the earth through the processes of wet and dry atmospheric deposition. When that happens, the fine particles can contribute to serious respiratory illnesses, such as asthma and chronic bronchitis, and can lead to premature death. Furthermore, SO2 acidifies surface water, reducing biodiversity and killing fish; damages forests through direct impacts on leaves and needles, and by soil acidification and depletion of soil nutrients; contributes to decreased visibility (regional haze); and speeds weathering of monuments, buildings, and other stone and metal structures.
Particulate matter (PM) is the term used for a mixture of solid particles and liquid droplets found in the air. The EPA classifies fine inhalable particles as anything 2.5 microns (millionths of a meter) or smaller in diameter, which it calls PM2.5. Although power plants are allowed to emit a very limited amount of PM directly into the air, one of their major contributions to PM air pollution is from the emission of SO2, which is converted into sulfate in the atmosphere and can be transported for hundreds of miles. The EPA has said the health effects of this PM can include:
- ■ Increased premature deaths, primarily in the elderly and those with heart or lung disease.
- ■ Aggravation of respiratory and cardiovascular illness, leading to hospitalizations and emergency room visits in children and individuals with heart or lung disease.
- ■ Decreased lung function and symptomatic effects, such as those associated with acute bronchitis, particularly in children and asthmatics.
- ■ New cases of chronic bronchitis.
- ■ Increased work-loss days, school absences, and emergency room visits.
- ■ Changes to lung structure and natural defense mechanisms.
Regulations Lead to SOx Emissions Reductions
In June 1989, President George H.W. Bush proposed sweeping revisions to the Clean Air Act. Building on congressional proposals advanced during the 1980s, Bush proposed legislation designed to curb three major threats to the nation’s environment and the health of its people: acid rain, urban air pollution, and toxic air emissions. The proposal also called for establishing a national permits program to make the law more workable.
By large votes, both the U.S. House of Representatives (401–21) and the U.S. Senate (89–11) passed clean air bills that contained the major components of the president’s proposals. A joint conference committee met from July to October 1990 to iron out differences in the bills, and the package recommended by the conferees was overwhelmingly approved by Congress. The president received the bill on Nov. 14, 1990, and signed it the next day.
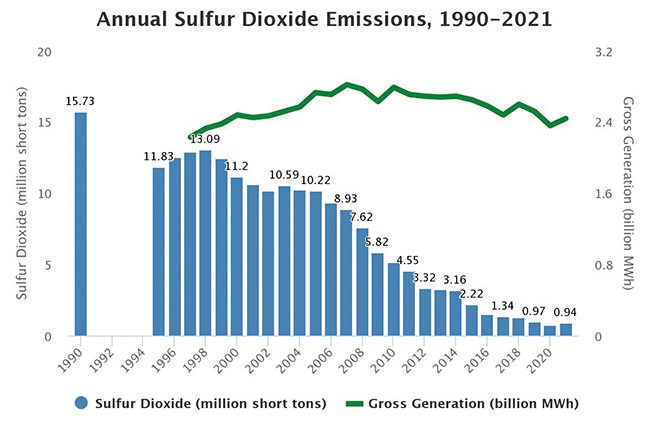
SO2 emissions from U.S. power plants began decreasing soon after the 1990 Clean Air Act Amendments (CAAA) were enacted (Figure 1). Among the provisions authorized by the CAAA was the Acid Rain Program (ARP), which imposed a cap on emissions of SO2 from coal and residual-fuel oil-fired power plants starting in 1995. The program was primarily motivated by concerns regarding acid rain affecting areas downwind of plants emitting SOx compounds. Because coal-fired units accounted for a large share of SO2 emissions, the program provided an economic incentive for them to install pollution control systems, burn lower-sulfur coal, and for high-emitting plants, dispatch less electricity.
 |
|
1. From 1995 to 2021, annual emissions of sulfur dioxide (SO2) from power plants fell by 92%. In 2021, sources in both the Cross-State Air Pollution Rule (CSAPR) SO2 annual program and the Acid Rain Program (ARP) together emitted 0.94 million tons, a reduction of 11 million tons from 1995 levels. Source: U.S. Environmental Protection Agency (EPA) |
Other regulations also played a big role in further reducing SOx emissions. In 2005, the EPA developed the Clean Air Interstate Rule (CAIR), a cap-and-trade program intended to reduce SO2 emissions in the eastern half of the U.S. beyond the levels defined by the ARP. The CAIR addressed regional interstate transport of contributors to ground-level ozone (smog) by requiring 27 eastern states to file implementation plans to reduce SO2 emissions.
The 27 states regulated under the CAIR accounted for more than 80% of both coal-fired electricity generation and national SO2 power sector emissions in 1997. The CAIR, at least in part, led to 91 GW of coal-fired power capacity being retrofitted with flue gas desulfurization (FGD) scrubbers between 2005 and 2011. In fact, by the end of 2011, 60% of the U.S. coal fleet had FGD scrubbers installed. Additional state requirements and settlements under the CAA’s New Source Review program also contributed to an increase in coal plant environmental retrofits. The CAIR was replaced by the Cross-State Air Pollution Rule (CSAPR) in 2015.
Meanwhile, the Mercury and Air Toxics Standards (MATS) was finalized in 2012 and required compliance by April 2015 (or April 2016, with the approval of a one-year extension). Although the MATS did not directly regulate SO2, the main compliance approach implemented by many electric generators was to install FGD scrubbers or dry sorbent injection equipment—both of which also remove SO2 in addition to the targeted air pollutants regulated under MATS.
SO2 emissions have also been reduced through various operating strategies and adoption of certain combustion best practices across the coal fleet. Among other factors affecting emissions reductions has been a general movement away from coal-fired power, as public perceptions concerning its negative effect on climate change has gained momentum; closure of smaller, less-efficient coal units, which had relatively higher SO2 emissions profiles; a reduction in coal power dispatch due to an abundance of low-cost natural gas providing gas-fired units with a competitive advantage; and similar effects caused by the growth of renewable energy resources.
How FGD Systems Work
There are two common FGD scrubber technology options typically employed for removing the SO2 produced by coal-fired power plants. They are limestone forced oxidation—a wet FGD technology—and lime spray dryer—a semi-dry FGD technology that employs a spray dryer absorber (SDA).
In wet FGD systems, the polluted gas stream is brought into contact with a liquid alkaline sorbent (typically limestone) by forcing it through a pool of the liquid slurry or by spraying it with the liquid. The alkali reacts with the SO2 gas to form calcium sulfite (CaSO3) or calcium sulfate (CaSO4), which is collected in a liquid slurry form. The CaSO3 and CaSO4 are allowed to settle out and the majority of the water is recycled. The settled material, called FGD scrubber material or scrubber sludge, is an off-white slurry with a solids content of about 5% to 10%. Because coal-fired power plants typically have both an FGD system and a fly ash removal system, fly ash is sometimes incorporated into the FGD material.
In dry FGD systems, the polluted gas stream is brought into contact with the alkaline sorbent in a semi-dry state through use of a spray dryer. Dry FGD systems use less water than wet systems and produce a dry by-product. The most common dry FGD system sprays slaked lime slurry into the flue gas. The main product of dry FGD systems is CaSO3 with minor amounts of CaSO4. The removal efficiency for SDA systems decreases steadily for coals with an SO2 content exceeding 3 lbs SO2/MMBtu; therefore, the technology is generally only used in plants that have the option to burn coals with sulfur content below that threshold.
FGD scrubbers are capable of reduction efficiencies in the range of 50% to 98%. The highest removal efficiencies are achieved by wet scrubbers, often greater than 90%. The first dry scrubbers to enter service had efficiencies of less than 80%, but the technology has evolved and the newest designs are capable of higher control efficiencies—on the order of 90%.
Among the disadvantages of FGD systems are:
- ■ High capital, and operations and maintenance (O&M) costs.
- ■ Scaling and deposit of wet solids on absorber and downstream equipment.
- ■ Wet systems generate a wet waste product and may result in a visible plume.
- ■ Cannot be used for waste gas SO2 concentrations greater than 2,000 parts per million (ppm).
- ■ Disposal of waste products significantly increases O&M costs.
 |
|
2. From 2000 to 2021, fine particulate matter (PM2.5) concentrations decreased 37% based on data from 375 trend sites monitored by the EPA. Source: EPA |
Still, the environmental and health benefits are substantial. The use of FGD systems has significantly reduced SOx and PM2.5 emissions from U.S. power plants. Using a nationwide network of monitoring sites, the EPA tracks ambient air quality trends for PM. From 2000 to 2021, average PM2.5 concentrations decreased 37% nationally based on data from the 375 trend sites the EPA monitors (Figure 2). As for SO2, from 1995 to 2021, annual emissions of SO2 from power plants fell by 92%.
—Aaron Larson is POWER’s executive editor.