Coal Plant O&M: River Locks and Barges Are an Aging Workforce, Too
During 2005, about 150 million tons of coal were transported to power plants by hopper barges plying U.S. inland waterways. With coal-fired plants expected to continue producing 50% of America’s electricity, coal barge traffic is not likely to fall off. In fact, it may increase, for two reasons.
One is cost. Shipping coal by barge (Figure 1) is cheaper than sending it by rail. According to Daniel T. Martin, a senior VP for Ingram Barge Co., barges account for 15% of total U.S. shipped coal tonnage, but those shipments produce just 5% of the industry’s freight costs. Martin says the barging of coal reduces stress on U.S. highways and railroads — a big benefit for communities concerned about crowded roads and busy rail crossings.

1. More than 150 million tons of coal were transported to power plants by barge in 2005. Courtesy: Ingram Barge Co.
The second reason that coal barge traffic may increase has to do with changes in power plant coal sourcing. With the ongoing depletion of central Appalachia’s reserves of compliance (low-sulfur) coal, more Western coal will be needed to take up the slack. Some of it could end up on barges heading east. At the same time, continuing and widespread installation of scrubbers will increase demand for coal from the Illinois Basin and northern Appalachia, which are near plenty of navigable rivers.
Slower Rollin’ on the River
Satisfying this growth in demand for barged coal will be difficult, considering the delicate condition and advanced age of the U.S. waterway lock and dam infrastructure. Over the past 15 years, there has been a steady rise in the annual duration of total "unscheduled outages" of navigation locks (Figure 2). The unexpected failures should actually be expected, given that lock maintenance budgets have changed little recently. A recent U.S. Army Corps of Engineers study estimates that $600 million would be needed to clear up its backlog of lock maintenance tasks.
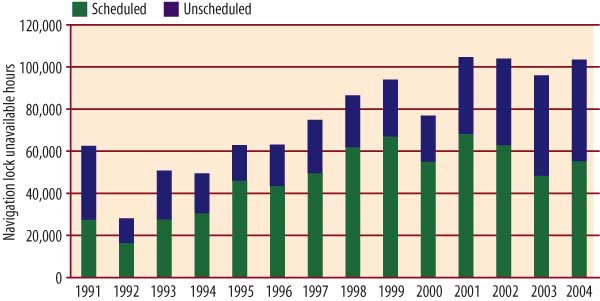
2. Unscheduled outages of navigation locks have become more frequent and longer in recent years. Source: Ingram Barge Co.
Every year seems to reveal a problem that is the tip of an iceberg. In 2003, for example, the Corps closed the main chamber of Greenup Lock, near Huntington, W.Va., for what was scheduled to be 19 days to repair the lock gates. However, after de-watering was complete and repairs commenced, it became clear that much more extensive work was required. All told, the lock was out of service for 52 days.
During this period, vessels had to wait an average of two days to pass through the lock through its smaller auxiliary chamber. According to Martin, the closing caused cumulative delays of 27,000 hours — a total of six years of normal operations — and an estimated financial impact of $41.9 million.
The next year, as another example, the McAlpine Lock and Dam near Louisville, Ky., experienced a scheduled shutdown of 11 days. Because this facility has no auxiliary lock chamber, the entire Ohio River came to a standstill. In this case, the Corps notified carriers, giving them time to reschedule and reroute shipments. Although the outage was planned, its financial toll on carriers forced to use higher-cost means of transportation was estimated at $4.5 million.
Although a handful of recently completed infrastructure projects have made coal deliveries more reliable and faster, budget constraints have prevented the Corps from addressing a host of what it calls "urgent" needs. According to Martin, the barge industry pays a user tax into the Waterways Trust Fund, which pays half the costs of infrastructure construction and upgrade projects.
Unfortunately, the Fund’s financial foundation is just as shaky as that of Social Security. It has a current balance of about $370 million and takes in about $90 million a year in user taxes. However, the Inland Waterways Users Board has identified "needed" infrastructure upgrades that will cost an estimated $1.6 billion over the next decade. That the math doesn’t add up is one more reason for accelerating investments in inland waterway infrastructure that have traditionally paid for themselves fairly quickly.
You Scratch My Back…
Navigation locks and dams aren’t the only pieces of U.S. coal’s aqueous transportation infrastructure that are aging fast. So, too, is the barge industry’s "floating stock." About one-third of today’s 17,000 hopper barges are scheduled to be retired by 2010 (Figure 3). Replacing them will cost $2 billion to $3 billion.
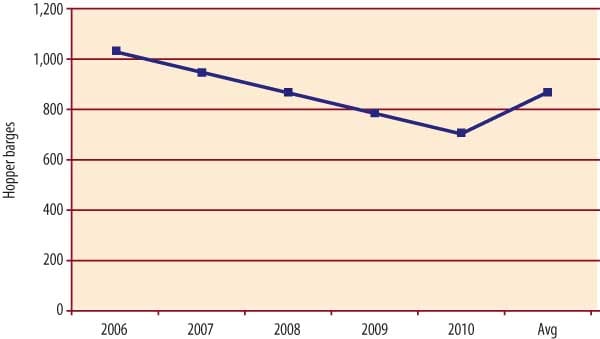
3. As hopper barges are retired, major investments will be required to replace them. Source: Ingram Barge Co.
According to Martin, the U.S. barge industry will be more than willing to spend that sum on new assets. But he says his industry would like a little help from its customer — the U.S. power generating industry — in lobbying the federal government to raise that portion of the U.S. Army Corps of Engineers budget devoted to building new and upgrading old inland waterway infrastructure.
Martin says utility lobbyists should take every opportunity to remind elected officials of the role that coal barging plays in keeping their constituents’ electric bills low. With the help of targeted congressional appropriations, the inland waterways could be upgraded to move even more coal (and other cargo) cost-effectively. Without additional investment, Martin warns, expect more crippling, unscheduled outages such as the one at Greenup Lock. Sooner or later, the effects of delayed shipments on power producers will be higher wholesale production costs and higher retail rates.
— This item is based on a presentation given by Martin at the 2006 ELECTRIC POWER Conference & Exhibition.
New Baghouse Designs for High-Sulfur Coals
Many U.S. power plants are considering installing fabric filter baghouses to treat the flue gas produced by burning medium- and high-sulfur coals. The retrofit projects are all but mandated by the need to comply with increasingly stringent limits on emissions of particulates and mercury. In many instances the addition of a baghouse is accompanied by the installation of a selective catalytic reduction (SCR) system for NOx reduction.
When coal burns, over 99% of the sulfur it contains is converted to sulfur dioxide (SO2). Less than 1% is further oxidized into sulfur trioxide (SO3). An SCR system can convert an additional 1% to 2% of the SO2 to SO3 as the flue gas passes over the system’s catalyst. Downstream of the boiler and air heater, as the flue gas cools while passing through ductwork and equipment, any SO3 in it may combine with water vapor to form sulfuric acid (H2SO4).
Sulfuric acid is formed and condenses as it reaches the sulfuric acid dew point. As the sulfur content and the moisture in the coal increases, so does the acid dew point. The table illustrates the expected sulfuric acid dew points for a variety of coals, with and without an SCR system in operation. The increase in acid dew point can be even more dramatic if petroleum coke (pet coke) is co-fired along with coal.
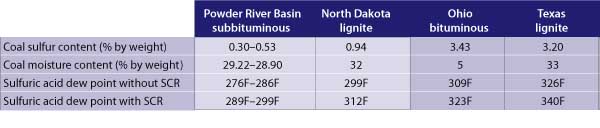
Sulfuric acid dew points for several coals, with and without a selective catalytic reduction system in service. Source: Sargent & Lundy
Some Don’t Like It Hot
Baghouses that treat flue gas from combustion of high-sulfur coal must be designed and operated correctly. A well-designed and -operated baghouse will perform well, with little maintenance, for many years.
A key tenet of baghouse design is to ensure that its inlet temperature (the outlet temperature of the air heater) is at least 25 to 40 degrees F above the maximum expected acid dew point. Another is to ensure that there is sufficient mixing in the flue gas stream from the air heater. This minimizes temperature stratification.
Stratification can cause some of the low-temperature flue gas to pass through the filter bags. Over time, accumulated condensation of the gas will cause the bags to plug. If the plant operates for long periods at low load, it also may be necessary to consider partially bypassing the air heater. Doing so will reduce stratification in the ductwork and the baghouse.
Preventing heat loss is another key to minimizing or preventing acid mist condensation in a baghouse when high SO 3 and moisture levels are present. Normally, baghouse purchasers specify that the differential between the gas inlet and outlet temperatures be no greater than 10 degrees F. Any large heat loss will increase the possibility of acid gas condensing on baghouse walls.
Insulation should be designed to keep the temperature differential between the inlet and outlet gas streams within 5 degrees F. Because compartments in the baghouse are occasionally isolated for maintenance, it is important to minimize the heat loss from an on-line compartment to an off-line compartment.
The filter bags of units used to treat flue gas from high-sulfur coal combustion should be:
-
Resistant to higher-than-normal flue gas temperatures (>325F).
-
Chemically resistant to attack by acids.
-
Equipped with a relatively moist filter cake that can be easily removed by a pulse of air.
Most filter bags found in pulse-jet baghouses at utility power plants are made of polyphenylene sulfide (PPS). PPS filter bags can operate reliably and continuously at up to 375F, and in "normal" flue gas they exhibit excellent resistance to sulfuric acid. However, this temperature ceiling could be lowered by flue gas rich in SO2 and/or SO3.
Why? The acid that condenses on the PPS filter bag surface wets the filter cake, making it harder to properly clean. But that is not the case for PPS bags equipped with a polytetrafluoroethylene (PTFE) membrane. Although such membranes are costly, they give PPS bags better cake release properties and improve their particle-capture efficiency.
Steel Cage Match
The cages that support the bags of pulse-jet baghouses are typically made of carbon steel. Under normal service conditions, these cages suffer enough acid attack and corrosion to shorten their useful life to five or six years. As the cages corrode, they develop "burrs" that rub and eventually cut the filter bags. Flue gas rich in SO2 /SO3, with elevated sulfuric acid condensation temperatures, accelerates corrosion and further shortens the service life of the cages.
There are two ways to make a cage more acid-resistant: Build it out of epoxy-coated carbon steel or stainless steel. A carbon steel cage coated with an acid- and temperature-resistant epoxy will offer good resistance to acid condensation. Although it will last longer than a noncoated cage, it will still wear out and eventually require replacement.
The other option — making the cage out of 304 or 316 stainless steel — is more expensive. But it is worth it if excellent resistance to acid condensation and corrosion and the longest service life are important.
Protecting Tube Sheets
Most baghouse tube sheets (Figure 4) are subjected to the same level of acid attack as baghouse cages. That’s especially true for the clean side of the tube sheet above the bags, where there is no coating of flyash to absorb condensed sulfuric acid.

4. The tube sheets and hatches of a pulse-jet baghouse under construction. Courtesy: Sargent & Lundy
There are several possible approaches to make tube sheets less vulnerable to corrosion, including:
-
Increasing the sheet’s corrosion allowance during its design.
-
Using acid-resistant coatings.
-
Making the tube sheets out of advanced materials.
Industry experience indicates that a carbon steel tube sheet with an increased corrosion allowance still may not prevent gas from bypassing the filter bags after years of continuous baghouse operation. Accordingly, this approach is no longer recommended.
One way to protect the upper side of the tube sheet, which lacks a flyash barrier, is to coat it with an acid-resistant and/or temperature-resistant epoxy material. High-temperature epoxies can handle constant or repeated exposure to temperatures as high as 300F to 350F in dry conditions, and to temperatures as high as 250F to 300F in wet conditions.
With epoxy coatings, it is possible to have high-temperature resistance or chemical resistance, but not both. As the temperature rises, the chemicals become more reactive; the most chemically resistant epoxies will fail at elevated temperatures.
In addition to the loss of chemical resistance at elevated temperatures, another downside of using coatings is the physical wear and tear they suffer both as they are installed and when bags and cages are replaced. For these reasons, coating the upper portion of the tube sheet is not a recommended approach for making it less vulnerable to corrosion.
Constructing a tube sheet from 304 or 316 stainless steel, or another corrosion-resistant alloy, adds resistance to sulfuric acid without requiring an increase in corrosion allowance. Of course, stainless steel tube sheets cost much more than conventional carbon steel tube sheets. Even so, a stainless steel or alloy tube sheet is recommended for baghouses intended to treat flue gas from combustion of high-sulfur coal. Normal carbon steel, on the other hand, should be adequate for the casings and hoppers of such baghouses (Figure 5).

5. The pulse header and tube sheet of a pulse-jet baghouse under construction. Courtesy: Sargent & Lundy
To minimize the possibility of acid condensation and corrosion, it’s important that baghouse surfaces be preheated. Adding hopper heaters is a routine part of baghouse design. The heaters are even more important for baghouses with flyash/filter cakes rich in sulfur compounds. Not only do they maintain metal temperatures above the acid dew point, they also serve to keep the ash/cake hot. Because of the elevated potential for acid condensation from burning high-sulfur coal, hopper heaters will be required during both unit start-up and steady-state operation. They should be capable of raising the hopper temperature to 250F within four hours.
SO3 Mitigation
It has been shown, both in demonstration and operating plants, that injecting alkaline materials can reduce the concentrations of SO2 and SO3 in a flue gas stream. An alkaline injection system can be installed to remove SO3 from the flue gas stream entering the baghouse as well as from the ductwork or equipment upstream of the baghouse.
These alkaline injection systems have been installed and are operating at several coal-fired power plants. They are used primarily to reduce and/or eliminate the trailing "blue plume" associated with high levels of sulfuric acid mist in the flue gas. Reagents that have proven effective include magnesium hydroxide slurry, hydrated lime, limestone, sodium bisulfite, and Trona. These substances react with SO3 to form solid particles that are collected in the baghouse. The resulting reduction in SO3 prevents H2SO4 formation. (See March/April 2007 COAL POWER for additional articles on SO3 mitigation.)
However, the potential drawback to using an alkaline injection system is the need for larger baghouses to collect the additional material. There is also the possibility that the collected flyash may not be suitable for alternate uses, for example, as an additive during cement production.
Precoating Bags and Surfaces
A fabric filter’s chemical resistance to acid corrosion can be enhanced by precoating its bags and casing surfaces with an alkaline powder. Alkaline powder such as limestone, lime, or sodium carbonate will help during start-ups. These alkaline powders help to neutralize any initial acid condensation before a protective layer of flyash forms on filter bags, casings, and hoppers.
During an initial start-up, the precoating may be done by pneumatic injection of the alkaline material through a port near the entrance of the baghouse. If a compartment is being returned to service after maintenance, one or more of the ports in the hopper area can be used to introduce the alkaline powder. Start-ups and shutdowns are critical times for baghouses at coal-fired power plants, and they usually present the greatest likelihood for acid condensation and corrosion.
When a baghouse compartment is taken out of service, closing its inlet and outlet dampers may prevent acid from condensing within it. This step should be followed by a cleaning cycle to remove as much of the filter cake from the bags as possible. After several cleaning cycles, the outlet damper of the compartment can be opened.
Proper baghouse O&M extends the unit’s life and improves its particle removal efficiency. As mentioned, maintaining the air heater exit gas temperature above the sulfuric acid dew point reduces the potential impact of acid condensation. It is important for plant operators to monitor the pressure drop across the baghouse compartments frequently. Doing so reveals incipient bag failures, reduces pluggage, and verifies the operation and temperature of hopper heaters.
On-line inspection of boilers often includes the use of infrared or ultrasonic scanners/cameras to detect failing insulation and air in-leakage around insulation, doors, and dampers. Similar on-line inspection techniques should be applied routinely to baghouses as well. Early detection and correction of problems can eliminate costly maintenance repairs and performance upsets later.
— This material is based on a presentation by Paul S. Farber and David Sloat of Sargent & Lundy, and Matt Usher of American Electric Power, at the ELECTRIC POWER 2006 Conference & Exhibition.