Technique Generates Salinity Gradient Power and Cleans Wastewater
Exploiting the difference in salt concentrations between the freshwater runoff from river mouths at the point where they meet saltwater reservoirs such as seas and oceans to harness power isn’t a new thing. Salinity gradient power has been recognized since the 1950s. Its massive global potential was estimated in the 1970s on the basis of average ocean salinity and annual global river discharges at between 1.4 TW and 2.6 TW. The most prominent technique to exploit the salinity gradient at river mouths is called reversed electrodialysis (RED), and it basically entails letting a salt solution and freshwater flow through a stack of alternating cathode and anode exchange membranes. The chemical potential difference between the freshwater and saltwater generates a voltage.
In a new article in the journal Science, however, researchers at Pennsylvania State University argue that RED’s potential applications are limited to coastal areas, and are impractical, owing to the need for a large number of membrane pairs (a RED module with a capacity of 250 kW, for example, is almost the same size as a shipping container). But the researchers say that the RED process could be improved using salt solutions that could be continuously regenerated with waste heat (of more than 40C) and conventional technologies that would allow a much wider application of salinity-gradient power. One possibility is to use the method on water containing food waste, domestic waste, and animal waste—which, the researchers claim, could represent a 17-GW power capacity in the U.S. alone.
Their proposed technique essentially combines the use of microbial fuel cells—which use exoelectrogenic bacteria, or bacteria found in wastewater that consume organic material and produce an electric current—and RED to create what they call a “microbial reverse-electrodialysis cell” (MRC, Figure 6).
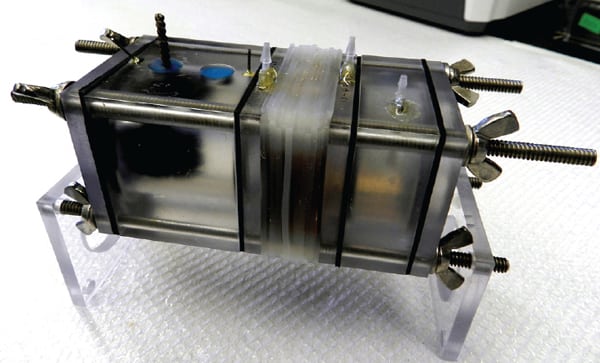 |
| 6. Power from salty water. A new technique engineered by Penn State researchers that combines bacterial degradation of wastewater with reverse electrodialysis—a method to extract power from a saltwater-freshwater gradient—promises to produce power anywhere. This image shows the researchers’ microbial reverse dialysis test cell, which produced 5.6 watts per square meter. Courtesy: Penn State |
MRC can work with natural seawater, but the organic matter in seawater will foul the membranes in RED stacks without extensive precleaning and treatment, the researchers found. So, rather than relying on seawater, the researchers used an ammonium bicarbonate solution, which mimics seawater but will not foul RED membranes. “The ammonium bicarbonate is also easily removed from the water above 110 F,” they suggest. “The ammonia and carbon dioxide that make up the salt boil out, and are recaptured and recombined for reuse.”
In tests using the ammonium bicarbonate MRC, the researchers reached a maximum power density (using acetate) of 5.6 watts per square meter of cathode surface area—five times greater than that produced using just the bacteria, and without a dialysis stack—and nearly 3 W per square meter with domestic wastewater. Maximum energy recovery with acetate reached 29.5% to 30.5%.
The researchers tested the MRC only in a fill-and-empty mode, but eventually a stream of wastewater could be run through the cell, they say. Not having to process wastewater was a major energy saver, said article co-author Bruce Logan, Kappe Professor of Environmental Engineering. “The bacteria in the cell quickly used up all the dissolved organic material,” he added. “This is the portion of wastewater that is usually the most difficult to remove and requires trickling filters, while the particulate portion which took longer for the bacteria to consume, is more easily removed.”
According to Logan, MRCs can be configured to produce electricity or hydrogen, making both without contributing to greenhouse gases such as carbon dioxide. “The big selling point is that it currently takes a lot of electricity to desalinate water and using the microbial desalination cells, we could actually desalinate water and produce electricity while removing organic material from wastewater,” he said.
The article authored by Roland D. Cusick, Younggy Kim, and Bruce E. Logan appears in the March 2012 issue of Science and is titled “Energy Capture from Thermolytic Solutions in Microbial Reverse-Electrodialysis Cells.”
—Sonal Patel is POWER’s senior writer.