In May 2006, the National Energy Technology Laboratory (NETL) of the U.S. Department of Energy’s (DOE) Office of Fossil Energy sponsored a two-session Environmental Controls Conference (ECC 2006) in Pittsburgh, Pa. The event was cochaired by Tom Sarkus, director of NETL’s Advanced Energy Initiatives Division, and Ron Cutright, director of NETL’s Major Projects Division.
The first ECC 2006 session focused on selective catalytic reduction (SCR) and selective noncatalytic reduction (SNCR) control of various nitrogen oxide (NOx) emissions. SCR and SNCR—the primary technologies currently available commercially for achieving significant reductions of NOx in flue gas—rely on reacting flue gas with a reagent (usually ammonia or urea) to produce environmentally benign nitrogen and water.
Conferences on SCR/SNCR have been organized and conducted by NETL nearly every year since 1997. The second ECC 2006 session addressed the related issue of reducing stack emissions and flue gas concentrations of sulfur trioxide (SO3). It was the second NETL meeting on that subject; the first was held in 1998.
SCR/SNCR working
Carl Bauer, NETL director, gave the keynote address of the SCR/SNCR session. He began by reminding the audience how much America relies on coal-fired power. The U.S.—which has 27% of the world’s proven coal reserves (an estimated 496 billion short tons, enough to last several hundred years)—currently gets about half of its electricity from coal. Because coal is forecasted as the cornerstone of America’s energy future, in partnership with industry, the DOE is developing advanced technology to provide cost-effective solutions to environmental concerns.
Based on past performance, there’s ample reason to believe that the technical community can deliver these solutions. SCR/SNCR technologies (among others) have proven capable of enabling any coal plant—new or existing—to operate as cleanly as a natural gas–fired plant, with respect to NOx.
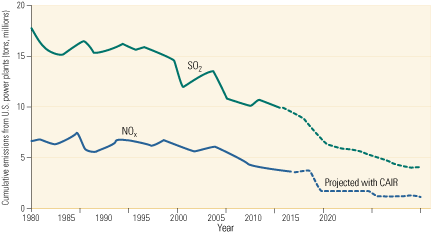
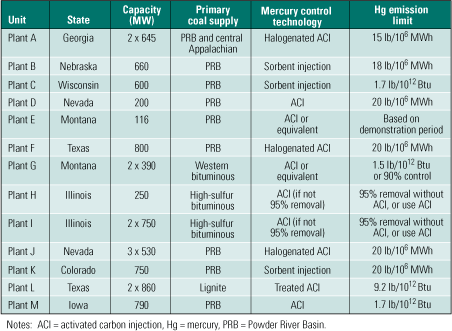
Since passage of the Clean Air Act in 1970, emissions have declined for virtually every pollutant monitored by the U.S. Environmental Protection Agency (EPA). In particular, sulfur dioxide (SO2) emissions have decreased 35% and emissions of fine particulate matter (PM10) have fallen 87% over the period, even as electricity production has risen 177% (Figure 1). The DOE has contributed toward this success by funding demonstrations of advanced SO2 scrubbers, a variety of NOx control technologies, air toxics and mercury (Hg) characterization studies, and clean-coal generation technologies. Building on this applied research, SCR systems will continue to be deployed domestically and worldwide.
2. CAIR builds on that progress. Significant advances in environmental protection by coal-fired power plants justify continued federal and state support for timely increases in coal-fired capacity. By 2020, the Clean Air Interstate Rule should have reduced nationwide emissions of SO2 from power plants below 5 million tons, and emissions of NOx well below that. Source: U.S. EPA
Tough row to hoe
At ECC 2006, Meg Victor of the EPA predicted that some areas of the U.S will have difficulty coming in under current caps on NOx emissions. As those caps are ratcheted down by implementation of the Clean Air Interstate Rule (CAIR), the Clean Air Visibility Rule (CAVR), and the future Clean Air Mercury Rule (CAMR), additional postcombustion controls on coal-fired plants will be needed, she added.
Complying with the progressively tighter limits of those three rules will require one of the largest investments in pollution control technology in history. The CAIR, CAVR, and CAMR standards will ultimately reduce SO2 and mercury emissions by more than 70% and NOx emissions by more than 60%, relative to 2003 levels (Figure 2). CAIR’s Phase II cap of 1.3 million tons/year on NOx will restrict power plant emissions of the pollutant to an equivalent limit of 0.125 lb/mmBtu. Significantly, the new limit will be applicable year-round, not just during the five-month summer ozone season, as is the current 0.15 lb/mmBtu limit on NOx.
2. CAIR builds on that progress. Significant advances in environmental protection by coal-fired power plants justify continued federal and state support for timely increases in coal-fired capacity. By 2020, the Clean Air Interstate Rule should have reduced nationwide emissions of SO2 from power plants below 5 million tons, and emissions of NOx well below that. Source: U.S. EPA
SCR operating experience
To kick off the SCR/SNCR session, Anthony Licata of Babcock Power Environmental Inc. and Clayton Erickson and Robert Lisauskas of Riley Power Inc. summarized the current status of SCR technology and projected likely future developments. Retrofits of older, smaller power plants may be more difficult to make, they said. To meet permitted limits, NOx levels may have to be reduced 90%, SO3 emissions will have to be cut, and releases of mercury will have to be minimized. Those challenges were discussed in subsequent papers.
Next, Jack Robinson Jr., PE, of SCANA summarized three years (16,000 hours) of SCR system operating experience at Wateree Station in Eastover, S.C. He reported that the separate systems for Units 1 and 2 have performed reliably and met all ozone-season performance guarantees—including 90% NOx removal, 2 ppm of ammonia slip at 3% excess oxygen, and 1.5% conversion of SO2 to SO3. The only major problem experienced was carryover of large particles of flyash, which adversely affected handling of the ash and the bags of each unit’s reverse-gas fabric filter. The problem was remedied by installing specially designed baffles in the economizers’ outlet duct.
Catalyst maintenance strategies
As SCR technology has matured, consultants, equipment vendors, and utilities have devoted major efforts to developing optimum strategies for maintaining (regenerating, cleaning, and replacing) catalysts. Catalyst maintenance was the subject of papers by Hans Sobolewski, Horst Rhein, and Hans Hartenstein of Steag LLC; Mark Schirmer of Cormetech and Mark Hill of TVA; Bill McMahon of SCR-Tech; Mike Cooper of SCR-Tech, Keith Harrison of Southern Company, and Chao Lin of American Electric Power; and Greg Holscher and John Cochran of Ceram Environmental Inc.
Concurrently, work is progressing on ways to determine SCR catalyst activity. They include in-situ measurement, which was covered by R. Smith and L. Muzio of Fossil Energy Research Corp., K. Harrison of Southern Company, D. Broske of EPRI, and C. Miller of NETL. Analytical procedures for catalyst testing were described by Riker Blank, Robert Becker, Dan Ott, and Andy Tobeck of Environex Inc.
SCR monitoring and control
Monitoring and control of SCR units is critical. Stephen Mandel and Richard Zuendt of Spectra Gases Inc. discussed instrumentation for measuring inlet and outlet NOx and ammonia concentrations. In a separate paper, Robert Romanosky and Susan Maley of NETL reported how their lab’s Advanced Research Program is supporting development of instruments and sensors suitable for the severe power plant environment. Some of these instruments are applicable to SCR process monitoring.
In a related effort, EPRI has devised a custom probe for use in hot gas streams with high particulate loading, as reported by Robert Spellicy of Industrial Monitor and Control Corp., Richard Himes, PE, of EPRI, and John Pisano of the University of California at Riverside. This technology has been tested for monitoring SO3 concentration in situ, and in real time, for the purpose of optimizing additions of alkali.
Charles Lockert and Bernard Breen of Breen Energy Solutions, Bryan Walsh of Duke Power, and Jacob Peter—an engineer at AES Corp.’s Cayuga Station—discussed a novel approach to SCR and SNCR performance evaluation: measuring the formation of ammonium bisulfate (NH4HSO4, or ABS), a by-product of NOx reduction.
Improving SCR performance
A novel way to enhance SCR system performance was described by D. Broske of EPRI and L. Muzio, D. Shore, J. Muncy, and T. Martz of Fossil Energy Research Corp. They explained that inter-layer mixing is very effective at maintaining uniform distribution of flue gas and reagent, markedly improving the efficiency of NOx reduction. Brad Adams and Connie Senior of Reaction Engineering International (REI) showed how computational fluid dynamics (CFD) can be used to model the various sections of an SCR system to optimize gas flow patterns, with beneficial impact on system performance.
The impact of SO3 formation on SCR systems serving units burning high-sulfur coals (a subject covered in greater detail in the SO3 session of ECC 2006 ) was discussed by Bill Ellison, PE, of Ellison Consultants (and one of POWER’s contributing editors), Volker Rummenhohl of TackTicks LLC, and Helmut Weiler of Weiler Consultants. They recommended using SCR catalysts with low SO2 oxidation rates, injecting alkali upstream of the air preheater, and a number of other measures to minimize corrosion.
The presence of large-particle ash (often referred to as popcorn ash) has become a serious concern for many operators of coal-fired boilers equipped with high-dust SCR systems. A paper presented by Hans Sobolewski, Hans Hartenstein, and Marilynn Martin of Steag, and Joseph Jancauskas and Michael Harrell of Dayton Power & Light’s J.M. Stuart Station, described the successful use of specially designed screens to continuously remove popcorn ash (see box).
New approaches to NOx control
Several alternatives to SCR and SNCR are being explored, with a view to reducing the overall costs of NOx reduction. One concept is to use a hybrid SCR/SNCR system, to reap the benefits of each technology. In one approach, Dale Pfaff of Fuel Tech Inc. and Richard Abrams of Babcock Power Environmental described the combination of a two-stage, urea-based SNCR system with in-duct SCR operation. Another hybrid system was proposed by Thomas Wright and James Cox, mechanical engineers with WorleyParsons Group.
A second approach, involving the use of carbon monoxide (CO) rather than ammonia as the reductant, was proposed by Zhen Fan and Andrew Seltzer of Foster Wheeler North America Corp., Richard Herman of Lehigh University’s Energy Research Center, and Kamalendu Das of NETL. In this novel concept, the reduction/oxidation (redox) reaction is catalyzed by a base metal catalyst, which absorbs SO2 as well as mercury. It has been tested on a bench-scale reactor, and further studies are under way using CFD simulations.
Two other papers described alternative approaches to NOx control. Both involve maximizing the use of layering (staging) and low-NOx burners and overfire air to minimize the load on the final reaction step. A paper by Charles Trippel, PE, and Peter Marx, PE, of Advanced Combustion Technology Inc.; Dick Carlow of Blue Ridge Paper Products; and Robert Durso of NRG Middletown Power LLC covered one approach.
The second paper was presented by Brad Adams, Marc Cremer, and Andrew Chiodo of REI; Craig Giesmann and Ken Stuckmeyer of Ameren; and John Boyle of Fuel Tech. The deep staging technology described in this paper makes extensive use of CFD, has been demonstrated on a commercial scale, and could prove capable of reducing NOx levels to 0.15 lb/mmBtu at a much lower cost than using SCR.
The ABCs of SO3
Bill Ellison gave the keynote address of the second ECC 2006 session, on SO3. He reviewed the high points of the 1998 NETL conference on sulfur trioxide as a way of showing progress since then.
Surprisingly, SO3 emissions from power plants rival those from chemical plants. During combustion, sulfur in fossil fuels is converted to SO2, most of which is removed by flue gas desulfurization (FGD) before it reaches the stack. While SO2 continues to be targeted for further reductions, SO3 also has become a target for additional control.
Minor amounts of SO3 are created by oxidation of SO2 not only in the boiler but also downstream in the air heater and over the catalyst in the SCR reactor. If the SO3 escapes, it can produce visible plumes and corrosive aerosol mists of sulfuric acid (H2SO4). FGD units cannot remove SO3 as readily as they can SO2. Plants burning medium- or high-sulfur coal that are equipped with wet FGD systems are particularly prone to experiencing stack opacity problems due to emissions of sulfuric acid. Making the situation even worse are the corrosion and fouling of heat exchangers that the acid fosters.
Fouling inside, opacity out
As Lewis Benson of Carmeuse North America Group and Bill Ellison explained in their paper and presentation, serious air preheater fouling by sticky, ABS-coated flyash can be prevented by efficient pre-removal of SO3, especially on high-sulfur units equipped with high-dust SCR systems. Depending on the composition of the coal being fired and the type and operation of the boiler, the average SO2-to-SO3 conversion rate in the boiler can be as high as 1.5%. Measured overall SO3 conversion rates across the SCR reactor may be as great as 4.5%, depending on the catalyst type and the reactor’s operating temperature.
In several German power plants, sulfuric acid concentrations of 32 ppm have been measured downstream of a high-dust SCR system. Some of this gaseous acid condenses onto cold surfaces (<280F) and is adsorbed onto gas-entrained flyash, producing sulfur-to-ash ratios as high as those of some U.S. bituminous coals. Accordingly, SO3 removal upstream of the air preheater minimizes air preheater fouling and reduces or eliminates opaque stack emissions from plants equipped with a wet scrubber.
Ways to reduce SO3
Edward Levy and his associates at Lehigh University’s Energy Research Center explained in a paper that SO3 formation in coal-fired boilers varies as a function of boiler design and operation and fuel properties. They have developed a chemical kinetic model for determining how much SO3 forms between the furnace exit and the SCR inlet. The model accounts for homogeneous gas phase reactions in the flue gas, catalytic formation on boiler tubes and on flyash, and depletion of SO3 by alkali compounds. The Lehigh program is pursuing ideas for mitigating SO3 formation via less-intrusive approaches than alkali injection.
Sterling Gray of URS Corp. and Mick Harpenau of Duke Energy’s Gibson Station in Indiana summarized experience with using SBS (sodium-based solution) injection (of Na2CO3 and/or NaHSO3) to neutralize SO3 emissions. The process has been applied commercially at six power plants with a combined capacity of more than 8,500 MW.
Design inlet SO3 levels range from 40 to 110 ppm. The reagent was injected upstream of the air preheater at three of the plants and downstream at the other three. Na2CO3 has proven to be the most cost-effective sorbent. At the Gibson Station, SBS injection (downstream of the heaters) has achieved 95% to 99% removal, with no visible plume opacity. The cost of removing the SO3 has been calculated at $350 to $500/ton.
In a separate paper (see Special Report), Rob Moser of Codan Development LLC—owner of the SBS Injection process—itemized and quantified SO3‘s impacts on unit performance and O&M. Reducing the level of sulfur trioxide to 3 ppm or less at the entrance to the air heater minimizes corrosion and fouling of back-equipment, enables additional heat recovery, reduces NOx emissions, and improves unit heat rate by a few percentage points—enough to save several hundreds of thousands of dollars annually.
Other papers on SO3 were by Keith Day and Jonathan Norman of O’Brien & Gere (injecting sorbents into ductwork), Lewis Benson of Carmeuse (injecting a slurry of magnesium hydroxide just upstream of the air preheater), and Bill Ellison and Lewis Benson (injecting hydrated lime). According to Benson, injecting Mg(OH)2 into flue gas to abate SO3 is a very low–capital cost retrofit process.
Final stats
ECC 2006 had about 290 attendees, including 14 visitors from 10 countries. Summaries and/or full versions of all of the papers mentioned above, as well as others, are posted at www.netl.doe.gov/publications/proceedings/06/ecc/index.html.