Battling White Rust
Does your power plant use a chiller for combustion turbine inlet air cooling or other processes that reject heat? If so, there is a good chance you also have an auxiliary cooling tower (Figure 1) or a wet surface air cooler (WSAC, Figure 2) to cool these systems. (See POWER, September 2008, “Wet Surface Air Coolers Minimize Water Use by Maximizing Heat Transfer Efficiency.”) Galvanized steel has been commonly used in the construction of smaller, package-type cooling tower systems because it is relatively inexpensive. These cooling towers are popular in many different industries and will increase in use in power applications.
 |
| 1. Cool breeze. This is a typical auxiliary evaporative cooling tower. Courtesy: Nalco Power ITC |
 |
| 2. Go with the flow. The co-current flow of air and water (both flow downward) in a wet surface air cooler increases the system’s heat transfer efficiency. Courtesy: Nalco Power ITC |
Those of us who work in the water treatment industry are familiar with the smaller, package-type cooling towers from our experience in the food and beverage, institutional, and manufacturing business units. These units are typically constructed using galvanized and stainless metallurgies. However, many in the power industry are only familiar with wood or fiberglass-reinforced plastic (FRP) because these are the materials most commonly used in the construction of field-erected cooling towers at central station power plants.
A cooling water treatment program must consider not just the galvanized, wood, or FRP but also all the materials that the water will come into contact with throughout the plant, such as titanium, stainless steel, and copper-based alloys used in the condenser and possibly other balance-of-plant heat exchangers.
The question of acceptable chloride levels in the bulk cooling water always comes up anytime stainless steel alloys are used. Typically, the engineering firm responsible for the plant design will select appropriate materials and a water chemistry program that ensure chloride levels in the cooling water are kept at acceptable levels, depending on the grade of stainless. When stainless steel is used, developing an acceptable water treatment program is straightforward.
Galvanized cooling towers are a much different story. Although rare in the power industry, towers using galvanized steel components are becoming more popular with the increased use of chillers for inlet air-cooling combustion turbines in combined cycle plants, as well as WSACs in the air-cooled configuration.
In general, all cooling towers are very sensitive to water chemistry, particularly chlorides, sulfates, and alkalinity. These constituents are often overlooked when designing a cooling water treatment program. (See POWER March 2013, “Selecting a Combined Cycle Water Chemistry Program” and September 2009, “Avoid These 10 Mistakes When Selecting Your New Water Treatment System.”) Even so, using galvanized parts within a cooling tower makes the problem of water treatment even more difficult—the water is tough on the galvanized parts, and the power industry operates cooling towers at much higher cycles of concentration than other industries.
Finally, the propensity for white rust formation in galvanized systems is not very well known in the power industry. The remainder of this article will define white rust, how it is formed, its potential impacts on equipment integrity, and how to control its formation.
Galvanized Steel and White Rust
Galvanized steel is carbon steel with a coating of zinc metal on its surface. Either a hot-dip or electroplating process can produce a zinc layer that is a protective surface coating as well as a sacrificial anode. Because zinc is less noble than carbon steel, it will corrode sacrificially to protect the underlying base metal. If the protective zinc layer should corrode through, the base-layer steel will corrode at the same rate as unprotected carbon steel. It is important to note that galvanized steel will always have some small amount of general corrosion occurring, just as with mild steel. The key is to minimize those general corrosion rates while minimizing the potential for localized corrosion.
When a galvanized steel system is operated under nonaggressive conditions such as a neutral pH and moderately hard water, a protective layer of nonporous zinc carbonate and zinc hydroxide forms to prevent further corrosion of the zinc coating. However, if the system is operated under aggressive conditions such as high pH, low hardness, or high chloride or sulfate levels, rapid corrosion of the protective zinc layer can occur. If the corrosion is severe, all the galvanized coating will be removed, exposing the unprotected steel, producing the familiar rusty-brown or black appearance. In less-severe cases, the zinc coating can be corroded to form what is known as “white rust” (Figure 3).
 |
| 3. Corrosion begins. White rust on a galvanized panel is caused when the protective zinc coating is attacked. When the galvanized surface is penetrated, the steel base metal will begin to corrode. Courtesy: Nalco Power ITC |
Both the protective oxide film and white rust are forms of zinc carbonate and zinc hydroxide. The difference between the two is the rate of formation and the density of the layer. Rapid formation of an oxide film produces a porous deposit that allows continued corrosion of the nonpassive surface, if left unchecked. A slower formation of the zinc layer provides a nonporous, protective layer.
A common misconception is that once the layer of white rust has formed, no further corrosion can take place. Not only is this not true, but the corrosion byproduct also can create an oxygen concentration cell, creating an accelerated localized corrosion reaction. This situation is further complicated with the addition of high levels of sulfates and chlorides. As stated in the Nalco Guide to Cooling Water System Failure Analysis (1993), “Once the zinc layer is breached, the underlying steel becomes susceptible to attack and is severely wasted locally” (Chapter 5, p. 108). The reference book also notes: “On galvanized steel, tubercles may develop rapidly at breaches in the zinc layer. Attack is frequently highly localized if aggressive ions such as chloride or sulfate concentrate beneath deposits” (Chapter 4, p. 72).
White rust has a porous, soft, gelatinous, or waxy appearance and is relatively easy to identify. The galvanized zinc coating can be severely damaged when white rust is formed, which in turn can shorten the life of the cooling tower. Also, because white rust formation can be highly localized, once the zinc layer has been consumed, corrosion of the mild steel may progress rapidly, as with any penetration of a metal coating. White rust is typically caused when operating outside of the typical guidelines for the cooling water pH and alkalinity. However, many cases of white rust have been attributed to improper passivation of a new or newly cleaned system, which is typically caused by compressed commissioning schedules and short-circuited cleaning steps.
White rust can quickly become a very costly problem. Not only does it cause heat exchanger degradation across the tube bundle during normal operation, but also cleaning and remediating a system with white rust can be a costly venture. Remediation typically requires mechanical brushing of the affected area, painting with an aluminum paint or epoxy coating, followed by a repassivation of the system. These steps may need to be repeated, depending on the severity of the white rust (BAC Technical Resource Document – RLD 1069–9/2010).
Preventing White Rust Formation
A better strategy for battling white rust is to take steps to prevent its formation in the first place. There are five preventive steps you should consider.
Choose a Different Metallurgy. The obvious first option would be to select a different alloy—one chosen specifically with the makeup water chemistry in mind. As more plants become reliant upon relatively aggressive makeup water from nonstandard sources such as high-conductivity well waters and municipal gray waters, the use of galvanized steel for cooling tower construction should start to diminish. However, one of the attractions to using galvanized steel towers is the often lower first cost, which may drive the purchase decision. If that is your situation, then other solutions must be explored.
Operate Under Manufacturer’s Guidelines. Though not popular in the power industry, galvanized cooling systems have been used extensively in other industries with very good success. The difference between success and failure can be as simple as following the manufacturer’s guidelines. Our experience has been that when users follow the manufacturer’s guidelines to the letter, they tend to not have white rust formation on their galvanized towers. However, those who deviate from the guidelines tend to suffer with either white rust in the wetted areas or sulfate and chloride attack in the wet-dry areas. This is particularly true of those users who operate with very high cycles of concentration.
Those in the power industry often overlook the fact that these package cooling towers tend to have a greater percentage of drift than field-erected cooling towers. When drift occurs in galvanized systems, the evaporated salts can accumulate on the external structure and attack the galvanized surfaces. The tube-bundle will appear very well protected, but the structure of the cooling tower is deteriorating.
Discovering the acceptable chemistry limits for white rust prevention can be a bit complicated. As Table 1 shows, the Cooling Tower Institute guidelines have a much higher limit for sulfates and chlorides than the three leading manufacturers of galvanized cooling towers (Table 2). Following the conservative, manufacturer guideline for your particular tower is the most prudent approach.
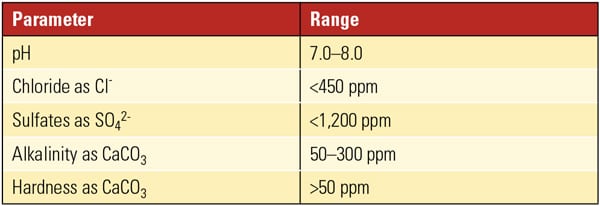 |
| Table 1. Typical cooling tower chemistry guidelines. Source: Cooling Tower Institute |
Complicating the decision, however, is the presence of two competing pH guidelines: operating pH for the passivation process as well as the manufacturer’s recommended operating pH for routine service (Table 2). The former guideline is important for two reasons. First, the initial passivation process seasons the galvanized steel, making it ready for normal service. The initial passivation is designed to allow for slow formation of the protective layer. Second, the passivation process can and should be repeated periodically if the tower is operated outside the manufacturer’s guidelines during normal operation. This is not to say the normal operating chemistry can be significantly outside the guidelines and still be reconditioned. Reconditioning does not restore the lost galvanized surface when severe damage to the zinc coating has occurred. Frequent visual inspection is recommended to ensure damage to the zinc coating caused by white rust formation is not taking place.
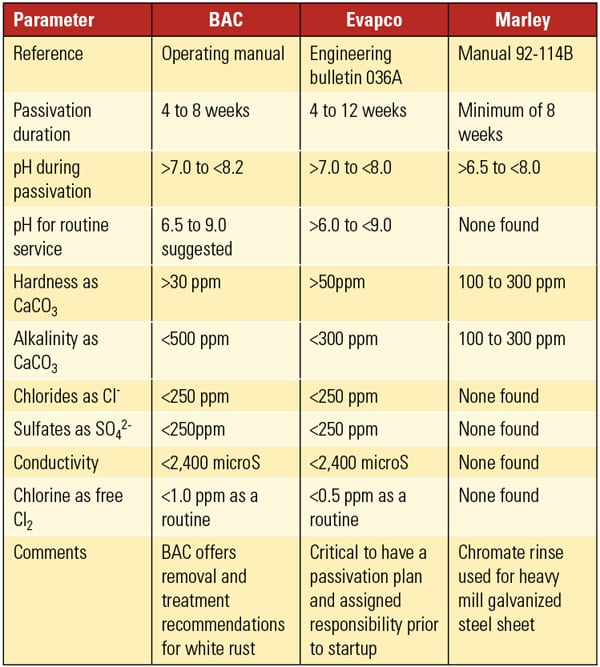 |
| Table 2. Galvanized cooling tower chemistry guidelines. Source: White Rust Prevention—An Industry Update and Guide (2012), Association of Water Technologies |
Coat the Galvanized Steel. If galvanized steel is to be used with an aggressive water treatment program, then coating the metallurgy may be an option to consider. For instance, BAC uses Baltibond, a special hybrid polymer that is impermeable to fluids. This material is applied by electrostatic spray to G-235 (Z700 metric) hot-dip galvanized steel surfaces. The initial and periodic seasoning of the system is not needed when this coating is applied. Although the coating will protect the structural surfaces, “hot-dip galvanized coils must use the water quality guidelines for galvanized steel units,” according to BAC Series V operating and maintenance instructions. In other words, the coating may prevent white rust formation on structural surfaces, but it will not prevent corrosion of the tube bundle. Therefore, the chemistry guidelines in Table 3 must still be followed.
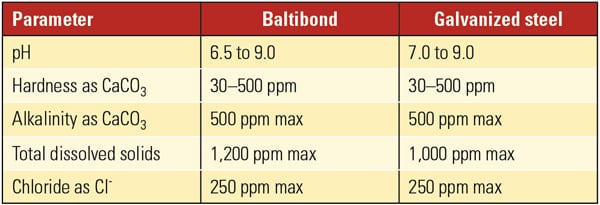 |
| Table 3. BAC chemistry guidelines. Source: BAC Series V and Low Profile V—Cooling Towers, Closed Circuit Cooling Towers, and Evaporative Condensers Operating and Maintenance Instructions |
Use Chemical Corrosion Inhibition. Until recently, there have been no water chemistry offerings to inhibit the formation of white rust. However, Nalco researchers have developed a chemical program that has proven to provide good protection for galvanized steel systems. Nalco 73801WR is a synergistic blend of passivation agents that can minimize white rust corrosion. Because NALCO 73801WR is specifically intended to inhibit white rust formation, it is designed to supplement other treatment programs such as mild steel corrosion inhibition and scale inhibition (Figure 4).
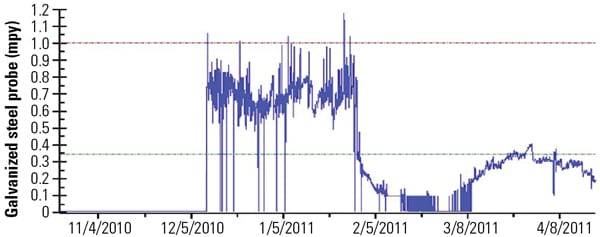 |
| 4. Good results. The initial test results of Nalco 73801WR have been excellent. This figure shows the results of a trial performed in a power plant closed-loop system. Source: Nalco Power ITC |
The corrosion rates for galvanized systems dropped significantly, from 0.75 mils per year (mpy) to 0.1 mpy after the initial feed of Nalco 73801WR on a test cooling tower. Although the corrosion rate rises and levels off at 0.25 mpy, that still translates into a 67% reduction in corrosion rate.
Another example involves a plant using a makeup water source high in conductivity, alkalinity, sulfates, and chloride, while operating at relatively high cycles of concentration for a galvanized cooling tower. This tower is also experiencing promising results using Nalco 73801WR (Table 4). This is a fairly new trial, so corrosion rates are not available yet. However, photos show excellent protection of the system (Figure 5).
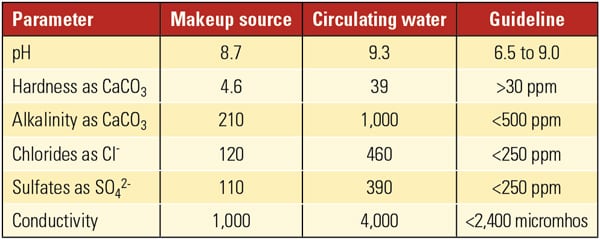 |
| Table 4. The chemistry of the makeup water and cooling tower bulk water are compared with the Association of Water Technologies guidelines summarized in Table 2. Source: AWT, Nalco Power ITC |
 |
| 5. Tube protection. Surface corrosion on galvanized tubes was prevented during recent testing. Courtesy: Nalco Power ITC |
Determine the Dose Rate. The typical dose rate for Nalco 73801WR is 50 ppm. However, halogen use rates and water conditions may dictate higher dose rates. Because there is no Trasar component in this chemistry, the additive dose rate must be slaved to the other chemicals used in the program.
The efficacy of the program will be, as with any corrosion-inhibition program, determined by monitoring corrosion rates. Monitoring for corrosion rates can be achieved via the following methods:
- Galvanized steel corrators measure current flow across two identical electrodes to which a small current is applied and measured. From the current flow, corrosion rates can be calculated and displayed online, from which trend lines can also be produced.
- Galvanized coupons of the same metallurgy can be used to produce absolute corrosion rates and the most accurate measurement of metal loss.
- Visual inspections may be subjective, but frequent inspections can prove to be very valuable in identifying problems before they become severe.
- Zinc coating testing is critical, as the coating is what you are protecting. Trend data collection is also important to help determine if the zinc coating is leaching into the system. A baseline should be developed for zinc levels in the system prior to introducing the program. Once the program is established, zinc levels should decrease if the program is working effectively.
Increased awareness of the potential pitfalls of using galvanized components in cooling towers is needed in the power industry. Though it is relatively inexpensive when compared to stainless steel, galvanized steel demands a certain diligence when developing a cooling water treatment program. Specifically, end users need to be aware of the makeup water source and the desired cycles of concentration in the cooling tower. Only then can they make an informed decision about a comprehensive water treatment regimen, including a corrosion inhibition plan, prior to startup.
—Kevin Boudreaux ([email protected]) is a power industry technical consultant for Nalco Co.’s Power Group.