High-Purity Boiler Water pH Measurement
Water quality monitoring and control are two of the most critical operations that power plants employ to protect assets, such as boilers and steam turbines, from corrosion by assuring the correct addition of chemicals. However, measuring the pH of ultrapure water can be maintenance intensive and costly. Yet, a strategy exists whereby a general-purpose pH sensor can be used to measure high-purity water successfully, significantly reducing time in the field, water usage, and costs.
The correct pH measurement is essential to water treatment: pH too high or too low can lead to boiler or steam turbine scaling and corrosion, system failures, and downtime, as well as the costly replacement of equipment.
For many low-pressure boilers, the use of a general-purpose pH instrument is straightforward, but for high-pressure boilers employing high-purity, low-conductivity water, such as those used in power applications where boilers and turbines require ultrapure water that is alkaline, unique challenges arise that can add costs, compromise accuracy, and threaten effective system operation. It is essential for users to understand these challenges when making decisions about the use of pH systems in high-purity water.
The Demanding Boiler
An important element of boiler feedwater treatment is controlling corrosion by keeping the boiler water slightly alkaline, between 7 and 9 on the pH scale. Alkaline pH causes an oxide film to form on the boiler tube surfaces that protects the base metal from further corrosion and allows breaks in the film to heal efficiently.
Control of the pH involves feeding sodium hydroxide and sodium phosphate salts in carefully administered quantities. Overfeeding chemicals, a common occurrence in most water treatment operations, can do as much damage as underfeeding, so continuous monitoring of pH is an important part of the boiler chemical control program.
But no pH sensor on the market can tolerate the temperatures and pressures found in even the smallest industrial boiler. Therefore, the pH sensor must always be installed in a cooled and pressure-reduced side-stream sample. To prevent flashing, the sample cooler must be located upstream from the pressure reduction valve.
Historically, the choice of a pH sensor for boiler water monitoring has depended primarily on the conductivity of the boiler water. Conductivity can range from 7,000 microsiemens per centimeter (µS/cm) in a low-pressure industrial boiler to under 10µS/cm in a high-pressure boiler in a steam power plant. A pH sensor that works fine in a low-pressure boiler has generally failed in a high-pressure boiler.
Choosing a sensor to measure the pH of high-conductivity boiler water is fairly straightforward. As long as the conductivity is greater than 50µS/cm at 25C and the suspended solids concentration is low, the use of a general-purpose pH sensor is ideal. The cutoff point between low- and high-conductivity boiler water is 50µS/cm, which is the lowest conductivity at which conventional pH sensors have reliably been used. Below 50µS/cm the sensor of choice has traditionally been one purpose-built to measure pH in low-conductivity water, but the trend is moving away from that approach.
Understanding pH Measurement
To better understand why measuring conductivity in high-purity water is so challenging, it’s useful to know a little about pH and pH systems. By definition, pH is the negative logarithm of the hydrogen-ion concentration in an aqueous solution. This means that a solution having a pH value of 4 has 10 times more hydrogen ions than a solution having a pH of 5.
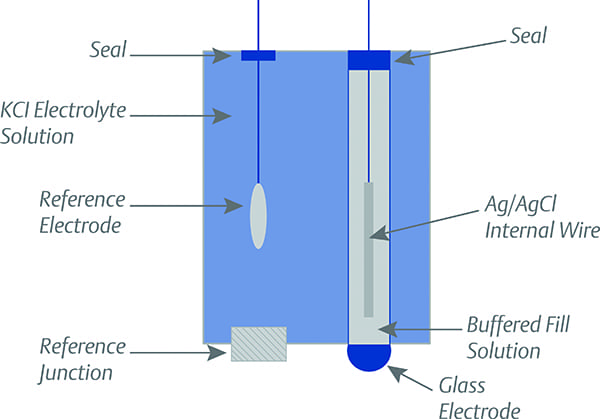 |
|
1. pH measurement is taken by comparing the electrical potential at two points: the reference electrode and the glass electrode. Courtesy: Emerson |
Basically, a pH control system measures the pH of the solution and controls the addition of a neutralizing agent (on demand) to maintain the solution at the pH of neutrality, or within certain acceptable limits. It is, in effect, a continuous titration. pH measurement is taken by comparing the electrical potential at two points—the reference electrode, which is surrounded by a constant pH electrolyte solution, and the pH glass electrode, which is in direct contact with the solution being tested. A reference junction is used to maintain the electrical connection between the reference electrode and the solution being tested (Figure 1).
High-Purity Water Challenge
The major challenge in measuring the pH of low-conductivity water is minimizing the difference between the liquid junction potentials in the calibration buffers and sample. The junction potential is the charge separation that arises when the reference electrolyte diffuses into the sample and the sample diffuses into the reference electrode. Electrolyte is critical to the proper functioning of the reference system for electrical connection, and ultimately, for the pH measurement, so when it runs out, the electrical connection between the pH electrode is broken and the measurement is impossible.
Ultrapure water accelerates diffusion of the electrolyte into the low-conductivity process water. The loss of electrolyte substantially shortens pH sensor life and increases the amount of maintenance required for each measuring point. Surprisingly, full diffusion can take place in the course of just a few months, requiring replacement of the pH sensor or a recharge of electrolyte fill solution.
An additional problem can arise when a pH sensor is calibrated because the junction potential that develops in the buffers becomes part of the calibration. If the junction potential in the sample is different, the mismatch will cause an error in the measured pH. The amount of error depends largely on the sample conductivity. Roughly speaking, above 20µS/cm the error is minor, but at lower conductivity the error can be as much as 0.5 pH. Clearly, such a margin of error is unacceptable when dealing with critical issues such as corrosion control. Because the mismatch is caused by diffusion of electrolyte into the process, it can be eliminated by using a flowing junction. The outward flow of reference electrolyte from the electrode effectively blocks the sample from entering the junction.
Traditional methods of dealing with high-purity water pH measurement issues have involved using a sensor with an electrolyte reservoir that continuously replaces the electrolyte. This approach, however, requires additional consumables to be ordered regularly, as well as stored, and also adds substantial ongoing maintenance.
A Less Costly Solution
With a range of advances in technology, this problem of high-purity water pH measurement can now be solved with the combination of a high-performance, general-purpose pH sensor that provides a solution ground to minimize drift and an isolated stable measurement, along with a low-flow controller. A general-purpose pH sensor can be used in this application if it offers certain critical characteristics that allow it to function in the challenging boiler water environment, which newer technology sensors can. The features needed include:
- ■ Advanced pH glass formulation that resists glass cracking.
- ■ A double junction reference, which protects the reference element from poisoning ions in the process.
- ■ A solution ground electrode that eliminates sensor drift caused by the mismatch between calibration buffers and low-conductivity fluid in the process, which is often seen in low-conductivity applications.
- ■ Smart pH circuitry that stores calibration information and enables auto sensor detection.
- ■ A sensor body that is rugged and chemically resistant.
- ■ No electrolyte (potassium chloride) replenishment required.
The other element of the system is a low-flow controller. Controlling the flow rate of water across the sensor directly, at a flow rate that’s held constant at less than three gallons per hour, reduces the rate of electrolyte depletion, increasing sensor life and reducing water usage by more than two-thirds versus other side-stream solutions. Water level must be maintained by an inner drain that keeps the water column, and in turn, the head pressure and flow rate constant. The transparent materials of construction make it easy to verify the continuous flow of sample through the flow path. When used with the low-flow controller (Figure 2), an advanced general-purpose pH sensor is able to respond to changes in pH at a minimum conductivity of 0.1µS/cm.
 |
|
2. Controlling the flow rate of water across the pH sensor directly reduces the rate of electrolyte depletion and increases sensor life. This is accomplished through the use of a low-flow controller, as shown in this image. Courtesy: Emerson |
The Accepted High-Purity Water Measurement Solution
A large power company in the southeastern U.S. is a classic example of the move toward measuring high-purity boiler feedwater with this combination of advanced technology general-purpose pH measurement and a low-flow controller. Like virtually all power companies, the utility treats its high-purity boiler water to a pH between 8.5 and 9.
For many years the company used a specialized high-purity water sensor to measure the pH. This sensor required refilling the reference solution. The initial price of this specialized sensor was high, and the cost of ongoing consumables and the maintenance required to keep the sensors filled was substantial. When the company learned of the general-purpose sensor alternative, it immediately recognized the potential value.
With the new approach, the company was able to settle on the same pH sensors it used for measuring cooling water and wastewater, which had the necessary solution ground, and added the low-flow controller. Saving money both in initial purchase and more in ongoing cost of ownership, the company has settled on the new approach as its standard boiler water pH measurement methodology.
Effective Results
A general-purpose pH sensor enhanced with flow controller technology can provide users with:
- ■ Four times the sensor life. With a general-purpose sensor design that includes an advanced glass formulation that resists cracking, as well as a double junction reference, users can expect sensor longevity of up to two years versus the industry average of six months.
- ■ 60% less time in the field and less maintenance time. General-purpose sensors often have smart pH circuitry built in to store calibration information and enable auto sensor detection. This kind of circuitry can make a sensor “plug and play” and cuts time in the field by more than 60%.
- ■ Two-thirds less water usage.
General-purpose sensors have a lower initial cost than specialized high-purity designs. In addition, their extended sensor life and reduced costs of maintenance and upkeep means the ongoing cost of ownership is also reduced. Further cost reduction is realized when the general-purpose sensor is combined with the low-flow controller, because unlike traditional high-purity sensors, ongoing consumables are not required. Finally, a long-life, general-purpose sensor plus low-flow controller requires less than a third of the water usage of other systems.
This new boiler water measurement methodology streamlines the measurement of high-purity boiler water, making it far easier for power plants to use—and afford. ■
—Michael Francis is a global product manager for Rosemount liquid analysis at Emerson (www.emerson.com/RosemountLiquidAnalysis).