Although the attention that SO3 has received has been focused on its visible emissions, the presence of SO3 also creates some serious operations and maintenance problems for back-end plant equipment, as detailed in Part I of this series. The effective removal of SO3 can eliminate those problems, producing some very valuable benefits for power producers.
The benefits of SO3 reduction
Although the O&M benefits achievable are a function of where and to what extent the removal occurs, for the purposes of this article, "effective SO3 removal" is defined as reducing its concentration to 3 ppm or less at the entrance to the air heater. (Figure 1 shows where SO3 is created and the forms it takes.) If this can be accomplished, the potential savings are considerable. Bear in mind that the examples presented here are merely illustrative; site-specific economic assessments would be required to provide quantifiable benefits for a particular plant.
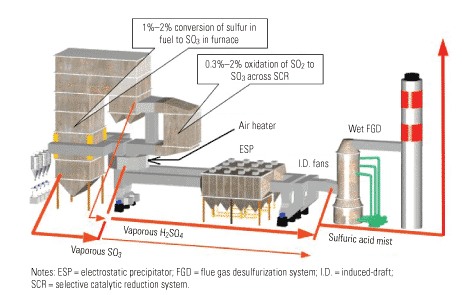
1. Acid reign. Where SO3 is created and the forms it takes. Source: Codan Development LLC
Reduced acid dew point and back-end corrosion. Limiting the level of SO3 at the entrance to the air heater to 3 ppm or less substantially reduces the sulfuric acid dew point. Figure 2 illustrates the relationship between the two variables. Note that at 60 ppm SO3—a concentration not unusual when burning a high-sulfur coal with a selective catalytic reduction (SCR) system in service—the acid dew point is about 310F. Also note that at 30 ppm SO3—a concentration typical when burning a high-sulfur coal without an SCR system in service or using an SCR system with a very low oxidation catalyst, or firing a medium-sulfur coal with an SCR system—the acid dew point is roughly 295F. At 3 ppm SO3, the acid dew point is about 255F.
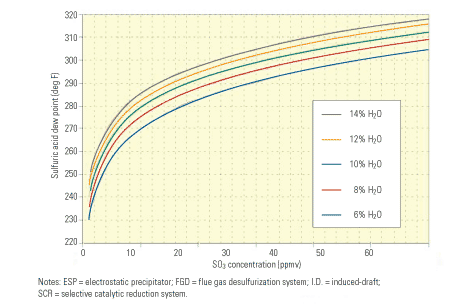
2. Dew diligence. The lower the SO3 concentration at the air heater inlet, the lower the sulfuric acid dew point and the lower the potential for corrosion of back-end equipment. With the unit operating at an excess air level of 8%, at SO3 concentrations of 60 ppm, 30 ppm, and 3 ppm, the corresponding dew points are 310F, 295F, and 255F, respectively. Source: F.H. Verhoff and J.T. Banchero, Chemical Engineering Progress, Vol. 70 (1977), p. 71
Because the SO3 gas will not condense until cooled to its dew point, the potential for corrosion can be reduced considerably by capping the concentration of SO3 at the air heater inlet at 3 ppm. And if it can be reduced to 3 ppm or less there, the SO3 level will almost certainly be reduced further as the gas is adsorbed on flyash in the air heater and on ductwork to the electrostatic precipitator (ESP). All told, corrosion of all back-end components will be greatly reduced.
Although the extent and severity of corrosion varies from plant to plant, the resulting damage is greatest to ductwork, the ESP, and the blades and housing of induced-draft (ID) fans. Repairing corrosion damage at every opportunity is important because corrosion fosters costly increases in air inleakage. It is not unusual for a utility to spend several million dollars every few years to repair corrosion damage and/or replace damaged components. Although the costs of corrosion damage also are very site-specific, the financial benefit derived from the effective removal of SO3 and the subsequent reduction of corrosion in back-end equipment could easily exceed $500,000 per year.
Reducing SO3 levels and corrosion potential also saves money by eliminating the need to modify and coat air heater baskets to protect them and make them easier to clean. It is reasonably common for power plants, when they add an SCR system, to modify the physical configuration of the air heater to allow better cleaning access to the areas where ammonium bisulfate (ABS)—a sticky solid—is prone to form and deposit.
Generally, two modifications are made. In one, three-layer basket designs are converted to a two-layer design and the basket at the heater’s cold end is made deeper. With this modification, the critical temperature range for ABS deposition then occurs in the cold-end basket, rather than in the intermediate layer, where cleaning access is limited and difficult to accomplish. The second modification is to coat the air heater’s cold-end baskets with enamel. This makes it more difficult for ABS to adhere to the surface and facilitates cleaning.
If SO3 is reduced to very low levels entering the air heater, it may be unnecessary to modify the air heater configuration and enamel-coat its cold-end baskets. One large coal plant reports having avoided an expenditure of $4 million to $5 million by eschewing these modifications. Although one would expect enameled baskets to be less vulnerable to corrosion and erosion damage, at several sites they have degraded due to the impacts of sootblowing and cyclic stress fatigue. What’s more, over the life of a plant, the cumulative cost to replace enameled air heater baskets may be twice as high as for traditional steel baskets.
Reduced heat rate and fuel costs. If the level of SO3 at the entrance to the air heater is reduced to 3 ppm (max), the heater can be operated with a lower outlet gas temperature. Doing so enables the heater to recover additional energy from the flue gas without increasing the downstream corrosion potential.
Benefits from the improved efficiency and the reduction in downstream gas volume can be considerable. Figure 3 compares the potential fuel savings achievable from reducing the SO3 concentration at the entrance to the air heater from the two typical levels of 30 ppm and 60 ppm. For each level, savings data are provided for three reduction levels: 80%, 90%, and 95%. This example is for a 500-MW unit with a heat rate of 9,500 Btu/kWh that burns a coal priced at $1.80/mmBtu and operates at an 80% capacity factor.

3. The more the merrier. The higher the level of SO3 in a unit’s flue gas before SO3 reduction efforts, the greater the potential fuel savings. The data are for a 500-MW unit with a heat rate of 9,500 Btu/kWh that is burning a coal priced at $1.80/mmBtu and operating at an 80% capacity factor. Source: Codan Development LLC
Two key assumptions are made here: that the air heater flue gas outlet temperature is reduced by an amount equivalent to the acid dew point suppression and that a change of 35 degrees F in that temperature equates to a 1% change in unit heat rate. As the bars in Figure 3 indicate, the annual fuel savings would be more than $750,000 per year for both cases of 95% reduction in SO3 concentration.
It is important to recognize that most air heaters would require their baskets to be modified to enable them to capture additional heat. The cost to do so is, once again, very site-specific. For example, the modification would be very expensive if it required taking the entire unit out of service or if the heater’s design necessitated internal, structural, or basket grid modifications. At the other end of the spectrum, the simple installation of tighter baskets (with more metal in the same volumetric area) during routine maintenance of the heater might raise costs by no more than 5% to 10%.
The modification’s impact on pressure drop also must be considered. If the elimination of ABS formation reduces fouling, the fan capacity that had previously been needed to compensate for pressure drop increases between planned outages would now be available to offset the higher pressure drop requirements of basket designs with a higher surface area or higher heat transfer coefficient.
It’s important to note that the shape of the dew point curves in Figure 2 bears heavily on the potential heat rate and cost benefits of SO3 reduction shown in Figure 3. Reductions in acid dew point (and, thus, heat rate) driven by reductions in SO3 concentration are significant only at very low levels. For example, the change in acid dew point between 60 and 30 ppm SO3 is about 15 degrees F—less than the 20 degrees F change in dew point produced by reducing SO3 from 10 ppm to 3 ppm. Accordingly, the heat rate/fuel saving benefits achievable are not very different for the 30- and 60-ppm cases, regardless of removal efficiency.
The amount of SO3 removed (and, presumably, the amount and cost of reagent required to do so) for a unit operating at a 30-ppm level would be one-half that for one running at 60 ppm SO3. The lower the concentration of SO3 in the target stream, the greater the savings potential. In other words, for units burning low-sulfur coal, savings are easier to come by. For example, removing 95% of the SO3 from a 10-ppm flue gas stream would suppress dew point by 40 degrees F (from 275F to 235F); at the same removal rate on a 60-ppm stream, the dew point would fall by 55 degrees F (from 310F to 255F). That means that 73% of the fuel savings of the 60-ppm case could be realized in the 10-ppm case with about 16% of the SO3 removal reagent cost.
At some plants, even greater savings are possible—if reducing the SO3 concentration at the air heater inlet to very low levels eliminates sticky fouling deposits typical of the interaction of flyash with SO3. This would allow the air heater to be operated at a lower outlet gas temperature, as described previously. More to the point, reducing this temperature reduces flue gas volume, with beneficial effects.
A reduction in operating temperature from 310F to 255F corresponds to a reduction of approximately 7% in gas volume. Reducing the amount of gas entering an ESP by this much improves its particulate collection efficiency in three ways:
- By increasing the ESP’s specific collection area by 7%.
- By increasing the efficiency of flyash collection (flyash resistivity falls with temperature).
- By decreasing the volume of flue gas that the unit’s ID fans need to handle, reducing their power consumption. The level of savings would depend on the type of drives the fans use and would be greatest for fans equipped with variable-frequency drives.
Reduced or eliminated ABS fouling of air heaters. ABS is formed when ammonia reacts with SO3 as flue gas cools in the air heater. Ammonia enters the flue gas as slip from the SCR or selective noncatalytic reduction (SNCR) system. If it is not removed upstream of the air heater, SO3, at a concentration of 30 to 60 ppm, can be a strong driver of ABS formation even if ammonia slip is low.
If SO3 can be removed effectively before the flue gas cools in the air heater, the potential for formation of ABS in, and fouling of, the air heater can be greatly reduced, if not eliminated. Doing so can significantly reduce or eliminate the need for unit derates or outages, as well as the cost of water washing to remove ABS deposits from the heater. The cost of element replacement also would be reduced because the factors that shorten element life (ABS and sulfuric acid corrosion, the frequency and pressure of sootblowing, and the frequency of water washes) would be significantly improved.
Higher NOx removal efficiency. Unless SO3 is minimized upstream, it is necessary to minimize the level of ammonia slip in the flue gas stream to prevent ABS from forming in the air heater. Note that minimizing ammonia slip reduces the NOx removal efficiency of an SCR system. Most systems call for no more than 2 ppm of slip, and some call for 1 ppm, max.
Allowing the ammonia slip to increase without compromising either the cleanliness of the air heater or the efficiency of the SCR system is possible only if the level of SO3 is minimized at the heater’s inlet. Raising the level of slip to, say, 4 to 6 ppm would allow the SCR catalyst to remove NOx more efficiently at a given catalyst activity level.
Maximizing the NOx removal efficiency of the SCR system maximizes generation of NOx credits, and revenues from their sales. Depending on the design of the SCR system, the removal rate could be increased by 5% or more. Figure 4 shows the revenues that would accrue to a 500-MW plant (with a capacity factor of 85% operating an SCR system year-round on a flue gas stream with 0.5 lb of NOx/mmBtu) from sales of NOx credits priced at $2,500 per ton. As the chart shows, increasing the SCR system’s NOx removal rate from 85% to 90% would make an additional $1.1 million in NOx credits available. That works out to a whopping $220,000 for each 1% increase in NOx removal efficiency.

4. Small gain, big bucks. Even a 5% increase in an SCR system’s NOx removal efficiency can translate into millions of dollars in revenue from sales of NOx credits. The data are for a hypothetical 500-MW plant with a capacity factor of 85% whose SCR system operates year-round on a flue gas stream with 0.5 lb of NOx/mmBtu of fuel. The NOx credits are assumed to be priced at $2,500 per ton. Source: Codan Development LLC
The benefits of raising the allowable level of ammonia slip go beyond revenues from sales of NOx credits. Doing so extends the life of the SCR catalyst before regeneration or replacement—both of which can be costly. Also, by raising NOx removal efficiency at lower catalyst activity, allowing a modest increase in ammonia slip pushes out the date when a unit outage will be required to regenerate or replace a spent catalyst. If that outage can be scheduled rather than forced, the impact on the unit’s bottom line will be enormous.
ABS formation caused by ammonia slip is even more critical for noncatalytic reduction systems. In SNCR, ammonia injected into the convective passes of the boiler reacts with NOx to form nitrogen within a very specific temperature range. Application of SNCR systems, which typically achieve modest (15% to 25%) rates of NOx reduction, has been limited to units firing relatively low-sulfur coal primarily to avoid the problem of ABS forming in, and fouling, the air heater.
If SO3 could be effectively removed before the heater, SNCR systems could achieve higher NOx removal rates (because higher levels of ammonia slip would be allowed) and warrant consideration for units burning higher-sulfur coals (because SO3 would not be an issue). Several hybrid technologies—for example, rich reagent injection (RRI), and installation of single-layer in-duct catalyst beds downstream of the SNCR injection point—are in various states of development and may prove capable of increasing removal efficiency and countering the negative impacts of ammonia slip on SNCR system performance.
Whatever the future holds, the benefits of increasing the NOx removal rate are the same whether SCR, SNCR, or hybrid technologies are used: Every 1% increase in the removal rate typically translates into $220,000 per year in revenues from sales of NOx credits for a 500-MW plant.
The previous analysis assumes that the plant already has an SCR or SNCR system in place. Clearly, if the capital cost of the systems were to be included, it would lead to a different conclusion. The lower capital costs of SNCR systems reflect their considerably lower NOx removal efficiencies. Theoretically, a power generator could substantially reduce its capital costs for NOx control by increasing the allowable ammonia slip and NOx removal rates of one or more of the SNCR systems in its fleet. If the increase in NOx reduction were sufficient, the generator might be able to avoid having to purchase an SCR system for one or more of its other plants. The capital cost savings potential of such a strategy and result would be in the tens of millions of dollars.
Reduced fireside corrosion. Many utilities minimize formation of NOx during combustion by using low-NOx burners, combustion staging, and/or overfire air systems. These techniques are effective, but they have been known to produce increases in unburned carbon in flyash and fireside corrosion.
For a unit equipped with an SCR system whose NOx removal efficiency has been increased by modestly increasing ammonia slip, reducing the combustion staging somewhat and allowing the SCR system to handle the additional NOx generated can be financially rewarding. For a 500-MW unit, decreasing unburned carbon in flyash by 1% results in a decrease of about 0.05% in fuel requirements. The annual saving would amount to about $25,000.
A saving of another order of magnitude would result from another consequence of reduced combustion staging—reduced fireside corrosion. Each and every tube leak caused by corrosion requires a unit to be shut down for repairs. In total, the costs of labor and materials for the repair and the lost revenues from generation sales can amount to $400,000 to $600,000 per incident, depending on the leak’s location and the boiler’s size.
Enabling the use of lower-oxidation catalysts. Suppliers of SCR catalysts have been working to lower the catalysts’ SO2 to SO3 oxidation rates. This would seem to be a good idea—as long as it has no downsides. Intuitively, one would expect that reducing the SCR system’s contribution to the SO3 level would make it easier to reduce that level to 3 ppm or less at the entrance to the air heater.
However, in the context of the O&M issues created by the presence of SO3, it is largely irrelevant whether the SCR system doubles the SO3 concentration or only increases it a few ppm. The same O&M issues will exist downstream of the SCR system whether the SO2 to SO3 oxidation rate of its catalyst is 0.2% or 2.0%. Only the magnitude of the issues will change. If SO3 can be effectively removed upstream of the air heater, it might make sense to consider the cost of doing so an economic tradeoff and, therefore, to reconsider the properties for which catalysts are designed.
The catalysts used in the first wave of deployments of SCR systems in the U.S. were designed to have high activity so they could achieve high NOx removal efficiencies. However, high SO2 to SO3 conversions—in the range of 1.5% to 2.0%—were not uncommon several years ago, as specified by original equipment manufacturers and architect/engineers. Over the past few years, much progress has been made in reducing the oxidation level to well below 0.5%.
Some of these specifications guarantee the SO2 to SO3 oxidation rate with or without ammonia injection. If an SCR system were to be operated without ammonia injection (that is, with the reagent system offline and no bypass), the oxidation rate surely would be higher than with it. The lower oxidation rates have reportedly been accomplished without compromising the NOx removal capability of the catalyst, which certainly is a very good thing. Moreover, low-oxidation catalysts will require either more surface area or greater volume to be able to be able to meet the efficiency spec, and that likely will raise their cost.
Over the past few years, another characteristic of a catalyst—its ability to effectively oxidize mercury—has become even more important than its oxidation rate. As part of their mercury strategies, many power plants that burn high-chloride bituminous coals now rely on their SCR systems to oxidize a very large fraction of the elemental mercury entering the system, facilitating the mercury’s removal by a wet flue gas desulfurization (FGD) system.
It would be presumptuous to conclude that a catalyst reengineered to reduce its SO2 to SO3 oxidation level would not suffer any impairment of its capability to oxidize elemental mercury. However, research is ongoing. By viewing the capability to effectively remove SO3 upstream of the air heater as an economic variable, catalyst suppliers could decouple the oxidations of SO2 and mercury when developing new catalysts. This could perhaps open the door to development of catalysts characterized by both high NOx removal and mercury oxidation rates—especially if mercury emissions credits become as tradable as SO2 or NOx credits.
Higher mercury removal efficiency. As mentioned earlier, SO3 can be adsorbed onto flyash; this is the mechanism by which SO3 reductions occur across air heaters and, to a lesser extent, in ductwork to the ESP. These same sites are equally capable of adsorbing mercury. Therefore, if there is still an appreciable amount of SO3 in the flue gas entering the air heater, after the gas cools the flyash entrained in it will be less able to retain mercury.
Figure 5 illustrates the effect of SO3 concentration at the ESP outlet on the retention of mercury by flyash at five different plants. In all cases, the fractional weight of mercury retained in flyash (normalized as loss on ignition, or LOI), increases as the SO3 concentration in the flue gas decreases. The effect is very dramatic when the SO3 concentration is very low (less than 5 ppm). This relationship is strong evidence that SO3 and mercury compete for the same adsorption sites on flyash.

5. Fight for that site. This graph shows the effect of SO3 concentration on mercury retention by flyash for five different plants. Across the board, the fractional weight of mercury retained (normalized to loss on ignition, or LOI), increases as the SO3 concentration in the flue gas decreases. The relationship strongly suggests that SO3 and mercury compete for the same adsorption sites on flyash. Source: Codan Development LLC
Retention of mercury by flyash may seem insignificant in the context of overall mercury removal strategies for high-chloride coals, which rely on SCR oxidation and subsequent removal by wet FGD to meet mercury emission limits. Yet capturing as much mercury as possible upstream of the wet FGD system may become more important in the future if mercury reemission by wet FGD and/or the fate of mercury in FGD gypsum emerge as constraining issues.
Without a doubt, however, the removal of SO3 upstream of the air heater can have some important implications for the use of activated carbon injection technology for mercury removal. It is reasonable to assume that both the mercury removal capability and the carrying capacity of activated carbon would be increased, perhaps quite appreciably, if SO3 were to be removed upstream of the carbon injection point by adsorption onto flyash. Furthermore, activated carbon has exhibited improved mercury removal and carrying capacity at lower gas temperatures.
If SO3 is effectively removed upstream of the air heater, it would be possible (as discussed above) to reduce the flue gas temperature without risking corrosion problems. If that is the case, it is likely that the performance and economics of activated carbon could be further improved. At this point, the cost of the activated carbon needed for mercury removal becomes a significant factor. Additional research is needed to better document and characterize any potential performance enhancements that this might provide. Yet it stands to reason that a reduction of 25% in the amount of carbon required for a 500-MW plant could easily produce annual savings well in excess of a million dollars.
Lower-temperature SCR operation. As noted earlier, SO3 can react with ammonia to form ABS under the catalytic conditions present in an SCR system (temperatures in the range of 530F to 630F). ABS can cause catalyst pluggage severe enough to warrant removing the catalyst for a period of heat recovery, possibly followed by its cleaning or replacement.
Although both the SO2 and SO3 levels in the flue gas are of significance here (because some of the former is formed by oxidation of the latter in the SCR system), the SO3 concentration in flue gas entering the SCR system is a more important factor in determining the minimum operating temperature or minimum ammonia injection temperature at which the SCR can be run without risking ABS formation. If the SO3 generated in the boiler can be effectively removed upstream of the SCR system, it may be feasible to lower its minimum operating temperature.
An SCR system that runs at a lower minimum operating temperature can be operated at lower boiler loads without the need for an economizer bypass to keep the temperature elevated above the minimum. Eliminating the need for an economizer bypass produces direct and substantial savings—possibly as high as several million dollars. In one fell swoop, the ability to operate the SCR system at a lower minimum temperature and at lower unit loads without an economizer bypass avoids degradations of unit efficiency at those times when the economizer bypass would have been in service and reduces the operating complexities and maintenance of high-temperature dampers, ductwork, and expansion joints.
Lower CO2 emissions. Any measures that improve the combustion efficiency of a power plant directly decrease its carbon dioxide emissions. Reducing the concentration of SO3 upstream of the air heater improves unit efficiency in two ways: by enabling increased heat recovery in the air heater and allowing for reductions in unburned carbon through reduced combustion staging. Additionally, if boiler-generated SO3 can be removed effectively upstream of the SCR system, use of the economizer bypass to control the system’s SCR minimum operating temperature can be avoided during low-load situations, preventing degradation of unit efficiency at those times.
Though there are currently no direct ways to quantify the value of reducing CO2 emissions, there is much talk of possible carbon taxes and/or CO2 emissions trading in the near future. Using the existing CO2 trading market in Europe as an example, at a current trading price there of $15 per ton of carbon dioxide, the value of a 1% reduction in CO2 emissions for a 500-MW power plant would be on the order of $600,000 per year.
To sum up . . .
It is nearly impossible to generalize about the total value of the benefits of SO3 reduction at any individual plant. Different situations will determine which and how many of the benefits described in this article are achievable, and their worth.
It is, however, safe to say that there are a number of specific items whose beneficial value alone could offset the entire cost of SO3 removal. It is very easy to conceive of situations in which the sum total of the benefit values would exceed the cost of removing the SO3 by many times.
—Robert E. Moser is a principal and cofounder of Codan Development LLC. The company’s patented sodium bisulfite SO3 removal technology is currently installed on over 8,500 MW of coal-fired capacity in the U.S. Moser can be reached at 831-438-0866 or fgdmoser@aol.com.