Regenerative activated coke technology (ReACT) is an integrated multipollutant control approach that removes SOx, NOx, and Hg from coal-fired plants by adsorption with activated coke to attain emissions levels found at natural gas–fired plants. One big advantage of this technology is that it uses only a fraction of the water used by conventional wet flue gas desulfurization. A recent license agreement brings this technology to the U.S.
Routine reductions in air emissions limits are a fact of life for power generators around the globe. In the U.S., adding wet flue gas desulfurization (wFGD) to remove SO2 and selective catalytic reduction (SCR) for NOx control are de rigueur for new plants, and these systems also have been retrofitted onto hundreds of operating coal-fired plants. The future will likely bring increasingly stringent control of criteria pollutants such as mercury and other hazardous air pollutants, plume visibility, disposal issues, as well as new greenhouse gas emission controls.
The conventional approach to controlling SO2 is by using either wFGD or a dry spray dryer absorber (SDA). Another option is regenerative activated coke technology (ReACT), an integrated multipollutant control approach that not only can remove SO2 and NOx from the flue gas stream but also removes mercury (Hg) through adsorption using activated coke. The activated coke sorbent is regenerated in the process while using only 1% of the water required by conventional wFGD systems. ReACT is fully commercial on coal-fired projects up to 600 MW in Japan and is now available in the U.S. through a recent license agreement between J-POWER Entech and Hamon Research-Cottrell Inc.
World-Class Performance
Japan’s Electric Power Development Corp. (EPDC) first negotiated pollution control permits for its 2 x 265-MW Isogo Thermal Power Station (Isogo) with the city of Yokohama City in 1964. EPDC launched a number of initiatives in the following years, including installing the first wFGD system in Japan.
 |
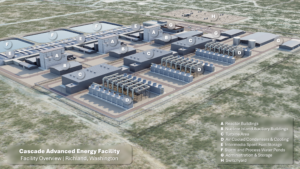
| 1. Best-in-class performance. The Isogo repowering project replaced two 265-MW conventional pulverized coal boilers and pollution control equipment (ESP and wFGD) with two 600-MW ultrasupercritical boilers fitted with ReACT advanced multipollutant controls. The newly repowered plant claims the title as the world’s lowest emissions-intensity coal-fired power plant. Design of the plant, located in Yokohama harbor, also considered plant aesthetics, including visibility of the stack. Courtesy: J-POWER |
After operating Isogo for more than 30 years, EPDC (now J-POWER) committed to replace the plant and negotiated with the city of Yokohama for a major repowering project, which would more than double generation capacity at the site yet further reduce emissions with more stringent environmental controls. The J-POWER/Isogo repowering project replaced the two vintage coal-fired units while more than doubling the generation to two 600-MW units within the same site limits. J-POWER’s newly repowered Isogo burns low-sulfur coal and incorporates high-efficiency ultrasupercritical boilers, low-NOx burners and controls, primary SCR, electrostatic precipitators (ESPs), and ReACT (Figure 1).
After more than eight years of operating experience at Isogo Unit 1 (the plant entered service in 2002), the ReACT system performance has been exceptional:
- Less than 5 ppm of SO2 at the stack with an SO2 inlet concentration of 200 ppm–400 ppm. SOx removal efficiency is over 98%.
- Greater than 90% mercury removal.
- An additional 20%–40% NOx reduction provided by ReACT as a co-benefit, dropping stack NOx concentrations to <10 ppm. An SCR is installed upstream as the primary NOx control.
Based on this record of Unit 1 performance, J-POWER agreed to a more stringent permit level for the second unit. Unit 2 began commercial operation in mid-2009.
Today, Isogo ranks as the cleanest coal-fired power plant in the world in terms of emissions intensity, with emissions comparable to those from natural gas firing. Isogo typically operates in the single-digit ppm concentration range for SO2 and NOx (against Unit 2 permit levels of 10 ppm and 13 ppm), at less than 5 mg/Nm3 (0.005 lb/mmBtu) for particulates, and with well over 90% control of both elemental and oxidized forms of mercury (Figure 2).
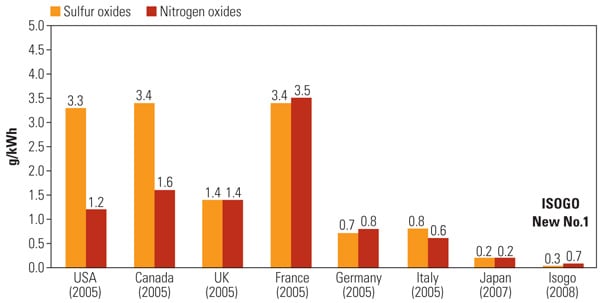 |
| 2. Small environmental footprint. ReACT is a regenerative activated coke–based technology that produces saleable by-products (sulfuric acid or gypsum) and, as a dry process, does not require water. The technology should be of special interest to U.S. utilities burning lower-sulfur western fuels, especially at locations where there are significant water supply concerns. J-POWER reports that SO2 and NOx emissions at the Isogo plant are both at single-digit ppm concentration levels—emissions that are on par with a natural gas–fired plant. Data for Japan represent combined data for Japan’s 10 regional electric utilities and J-POWER. Figures for Isogo represent actual performance for fiscal 2008. Country figures represent combined emissions intensity for coal-, oil-, and gas-fired thermal power plants. Source: Federation of Electric Power Companies of Japan |
Development History
The basic process concept originated with Bergbau Forschung in the 1950s in Germany. Additional development was provided by Foster Wheeler in the late 1960s in conjunction with its Resox process. Commercialization with activated coke was achieved by Mitsui Mining in the 1980s. Full commercialization by EPDC as an advanced generation multipollutant control technology for coal-fired boilers followed in the 1990s.
Process development efforts in the late 1970s focused on high-efficiency (98%) SO2 removal efficiency, but researchers quickly recognized that ReACT also provided NOx removal. The ReACT process design for SO2 control routinely removes 20% to 40% NOx as a co-benefit, and the process can be configured to achieve 50% to 70% NOx removal or higher by adding secondary injection of ammonia into the regenerator.
EPDC, which had faced serious difficulties in obtaining water from local government water agencies when siting earlier projects, began developing ReACT as an alternative to wFGD because the process used a fraction of the water required by a wFGD system. Reducing the water demand of the Isogo site post-repowering was a key project requirement, making ReACT a natural selection for that project. J-POWER subsequently acquired the rights to the technology from Mitsui in 2005 and has since provided units for refinery, incineration, and sinter plant applications through its subsidiary J-POWER EnTech.
The ReACT process has been successfully demonstrated in the U.S. over a period of five months as part of an Electric Power Research Institute project hosted by Sierra Pacific Power at its North Valmy Station. Valmy reported the following outstanding removal rates: 97.6% to 99.9% for SO2, 25.7% to 48% for NOx, and 97.1% to 99.6% for Hg removal. (The data were reported in “ReACT Process Demonstration at Valmy Generating Station,” C. Dene, et al., MEGA Symposium, Paper 123, 2008.) The expected high levels of SO2, NOx, and Hg removal were entirely consistent with commercial results at the full-scale units in Japan.
ReACT Process Description
The multipollutant control ReACT technology is a completely dry scrubbing system based on adsorption of SO2, SO3, and Hg and reduction of NOx to N2 on activated coke in a moving bed.
The complete ReACT gas scrubbing process requires three process stages, as illustrated in Figure 3.
 |
| 3. Three-step process. ReACT multipollutant removal is accomplished in three steps: The adsorption stage, where pollutants are captured on activated coke; the regeneration stage, where the activated coke is regenerated; and the by-product recovery stage, where a marketable sulfuric acid by-product is available. Source: Hamon Research-Cottrell Inc. |
Adsorption Stage. Downstream of primary particulate control, typically an existing ESP or fabric filter (FF), flue gas is contacted with a slowly moving bed of activated coke in pellet form as the process sorbent. This material’s properties are comparable to powdered activated carbon pellets (see table). The pellets are fed at the top of a cross-flow adsorber, where they come into contact with the flue gas. Activated coke removes SO2, SO3, NOx, Hg, and other species through adsorption, chemisorption, and catalytic reactions that are enhanced in the presence of ammonia. Adsorption does not consume water or lose water to waste disposal streams, and performance improves with lower back end flue gas temperatures. Particulates are also reduced across the moving bed by impact on the coke pellets.
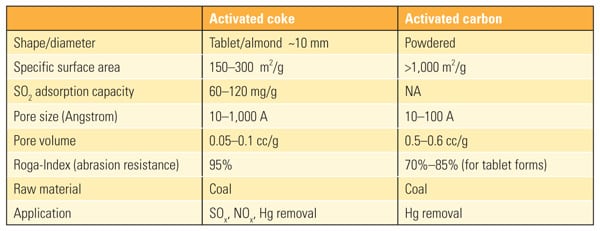 |
| Table 1. Comparison of the properties of activated coke and activated carbon. Source: Hamon Research-Cottrell Inc. |
The ReACT adsorber can be designed for up to 98% SO2 control levels. SO2 and SO3are adsorbed at high efficiency on the activated coke surfaces as sulfuric acid ammonium sulfate and ammonium bisulfate. The catalytic effect of activated carbon also leads to reduction reactions to reduce NOx in the presence of ammonia. Normalized gas space velocity of 300–500 hr-1 and activated coke residence time of 80–120 hr are typical. Ammonia is injected into the upstream flue gas at 0.5 mol ammonia to mol SO2. Contacting conditions in the adsorber provide 10 seconds for the flue gas to transit the high surface area available in the moving activated coke bed.
Mercury control in ReACT also occurs as a co-benefit from the SO2-based adsorber design. In the ReACT adsorber, the total carbon surface area in contact with flue gas, and contacting time, greatly exceed those of activated carbon injection methods practiced with an ESP or FF. Mercury capture at greater than 90% is achieved for both elemental and oxidized forms. ReACT also provides significant waste volume minimization for Hg-laden disposal materials.
Regeneration Stage. In the regeneration stage, pollutant-laden activated coke is transferred to a thermal regenerator, where reactions are completed and pollutants are desorbed as a sulfur-rich gas stream. There are no steam or SO3plume releases in this process. The regenerated activated coke stream is cooled, and fines are separated before being returned to the adsorber. Make-up coke in pellet form is added to replace the separated fines.
Activated coke at flue gas temperatures carrying adsorbed SO2, NOx, and Hg is fed through a lock hopper to the top of the regenerator (Figure 4). Activated coke moves downward by gravity flow through three indirect heat exchanger sections for preheating, heating, and cooling the regenerated coke, which is then discharged through a lock hopper to fines separation and returned to the adsorber. Non-isothermal kinetics related to thermal regeneration show that the thermal desorption is driven to completion by heating the activated coke to 450C.
 |
| 4. Temperature profiles. The heart of ReACT is the regenerator vessel, where the correct temperature profiles are maintained to maximize SO2, NOx, and Hg capture and removal. The activated coke process is completely dry and does not require water for reagent preparation or for humidification of the flue gas. Retention of adsorbed mercury in the regenerator allows the Hg to be held in place for controlled and reduced volume disposal on three- to four-year cycles. Source: Hamon Research-Cottrell Inc. |
Prior to cooling the activated coke to flue gas temperature for return to the adsorber stage, ammonia gas can be introduced to precondition the activated coke and provide increased NOx control.
Mercury compounds are also thermally desorbed at >400C to the vapor phase. This temperature is reached within the heating zone and mercury desorption is complete at the bottom of the heating zone.
Mercury and other desorbed gases flow upward and counter-current to the activated coke flow and contact lower temperature coke surfaces. At the top of the heating zone, the activated coke at 200C has effectively re-adsorbed all the mercury vapors.
By designing the adsorber in this way, the mercury is effectively confined in this zone of the regenerator. Because the capacity of activated coke for mercury adsorption is quite high, the Isogo plant extracts only 100 tons of activated coke during planned outages on two- to three-year intervals, or less than 0.1 ton per MW per year. Disposal is managed during a scheduled regenerator downtime (all activated coke has been cooled) by diverting the portion of activated coke that had been in the heating zone and lower sulfur-rich gas zone.
By-Product Recovery Stage. A sulfur-rich gas release zone is located between the preheat and heating sections of the regenerator tower. The sulfur-rich gas released (SO2, N2, CO2, and H2O) flows to an acid plant for the production of marketable sulfuric acid. Mercury in the sulfuric acid by-product at Isogo is reported at below detection limits. Power consumption of the ReACT is approximately 60% of a typical wFGD system.
Carbon Use and Disposal
In ReACT, granular activated coke is repeatedly recycled between the adsorption tower and the regeneration tower. The size of activated coke is gradually reduced over time from mechanical wear. Fines are separated prior to returning the activated coke to the adsorber. In addition, reactions in the regenerator use some carbon, and a make-up stream of activated coke replaces these losses. Operating results show that the activated coke supply rate is less than 1.5% of the circulating rate of activated coke. Because fines removal and other carbon losses are offset by make-up, the size of the activated carbon in the system reaches a stable long-term distribution.
Unlike other catalysts—which tend to deteriorate over time with activity loss due to aging, fouling, and contamination—the performance of the activated coke increases over time. Activity is improved through regeneration, which exposes fresh surface and micropores through carbon reactions and increases the number of functional groups on the catalyst surface from residual sulfur, oxygen, and nitrogen species. Regeneration of sorbent greatly reduces site logistics for reagent make-up processing and waste handling compared with other FGD processes.
Compare Performance to ACI Systems
A comparison of the carbon make-up and disposal requirements for a typical 250-MW plant using ReACT compared to wFGD limestone and to typical activated carbon injection (ACI) for Hg control illustrates the differences in incoming reagent quantities and mercury-laden material disposal. Consider the following comparison, which uses these assumptions:
- Basis: 250-MW PC boiler burning low-sulfur Powder River Basin coal, 75% capacity factor
- Firing rate: 2,800 million Btu/hr (at 11,000 Btu/kWh)
- Flue gas flow: ~1,000,000 acfm @ 300F
- Fly ash load: 2 to 3 gr/acf (55,000 tons/year @ 2 gr/acf)
- Hg load: 5 to 10 lb/1,012 TBtu
Limestone wFGD, which addresses primarily SO2 with some Hg co-benefits, for this typical plant would require about 15,000 tons/year incoming limestone and create approximately 25,000 of dewatered gypsum as a disposal stream.
For the ReACT process, fresh make-up activated coke replaces losses due to fines separation after regeneration and carbon consumed in regenerator reactions. Make-up for a 250-MW plant is approximately 1,250 tons/year (4 to 6 tons/year per MW), which addresses SOx, NOx and Hg control. Fines separation of the same 1,250 tons/year is in the form of mercury-free carbon, which can be burned for fuel value or may have beneficial reuse as a carbon sorbent at other sites.
For typical ACI systems that address Hg only, 90% mercury control requires injection of powdered activated carbon (PAC) into the flue gas at rates between 2 lb/million acfm and 10 lb/million acfm. The higher rates are generally associated with injection upstream of an electrostatic precipitator, and lower rates are associated with injection upstream of a fabric filter. Injection rates have some sensitivity to fuel type, boiler type, and the effects of increased SO3from an upstream SCR.
When PAC is injected upstream of the primary particulate control, whether it be an ESP or a FF, the fly ash is contaminated with mercury-laden carbon, which may render otherwise saleable fly ash for cement or road material into landfill waste or pose future questions as to waste disposal.
If activated carbon is injected into a secondary FF placed downstream of an ESP (which may be reduced to control the PAC/ash mixture at the FF to about 10% carbon), activated carbon usage can be minimized and fly ash beneficial use maintained, though with additional equipment and energy requirements. In this case, the disposal stream would be in the range of 6,000 tons/year for a mercury-laden carbon/fly ash mixture.
For a ReACT system serving a typical 250-MW coal-fired boiler, the activated coke from the mercury capture zone in the regenerator weighs about 30 tons and has a disposal rate less than 15 tons/year.
—H. James Peters (james.peters@ hamonusa.com) is executive vice president of products and technology for Hamon Research-Cottrell Inc.