Many combined cycle power plants have established a pH guideline between 9.6 and 10.0 to minimize corrosion in heat recovery steam generators and condensers. Neutralizing amines are used to achieve these pH targets. This article examines amine selection, the pros and cons of ammonia-only versus organic blends, the final fate of organic amines, and the quandary of feeding neutralizing amines.
Proper water chemistry is vital for combined cycle power plants to operate reliably. Without it, corrosion will damage systems and force unscheduled downtime. The following case study will help readers understand some of the challenges.
Defining the Problem
The subject facility is a 2 x 1 x 1 (two blocks of one gas turbine by one steam turbine) combined cycle power plant. Commissioned in 2016, the plant consists of two Siemens H-class gas turbines, with a total plant generating capacity of 830 MW. Steam is condensed by means of an air-cooled condenser (ACC). The heat recovery steam generator (HRSG) configuration is a standalone low-pressure (SALP) drum (64 psi), an intermediate-pressure (IP) drum (479 psi), and a high-pressure (HP) drum (2,390 psi with duct firing). Importantly, the cycle chemistry is an all-volatile, oxidizing program; no solid alkali (phosphate or caustic) is fed to the HRSG. A blended amine of ammonia and ethanolamine (ETA) has been used since construction, targeting a pH of 9.4–9.8. It is controlled using specific conductivity.
The author became involved with the subject plant when concerns arose about the level of amine usage, which was 22 gallons per day (gpd). Theoretically, it takes approximately 10 ppm of ammonia/ETA blend (depending on percent active) to achieve a pH of 9.6 in pure water. Assuming a condensate flow of 1.5 million lb/hr, this would equate to 43 gpd of chemistry. However, 95% to 99% condensate return is typical in a power plant. At this return percentage, 2.1 gpd should maintain a condensate pH of 9.6. The subject plant was using 10 times that amount, with condensate and LP/IP drum cation conductivities very high at 1.3 µmhos and greater than 5.0 µmhos, respectively.
A thorough investigation found much of the amine fed was being lost through the vacuum pump vent and overflow drain. After confirming amine was being lost via the vacuum pump knock-out tank, a plan to recycle the overflow drain back to the condensate receiver tank was developed. To eliminate the need for an additional pump and subsequent flow balancing, a slip stream was taken off the discharge of the heat exchanger forwarding pump. By throttling the slip stream, the knock-out tank level could be controlled, while also recycling amine.
The plan worked. Recycling reduced amine usage from 22 gpd to 3.5 gpd. Adding to that success, cation conductivities throughout the system dropped rapidly (Figure 1). However, all was not well with this approach. While amine usage appeared to be optimized, pH control throughout the HRSG appeared to have suffered. As Figure 2 shows, prior to modifications on August 23, the pH profile across the drums was consistent, averaging 9.6. After August 23, the HP drum pH increased, while the pH in the LP and IP drums decreased to dangerously low levels when considering flow-accelerated corrosion (FAC) potential. So, what was happening and why did it start after the modifications were made? This was where the discussion of neutralizing amines began.
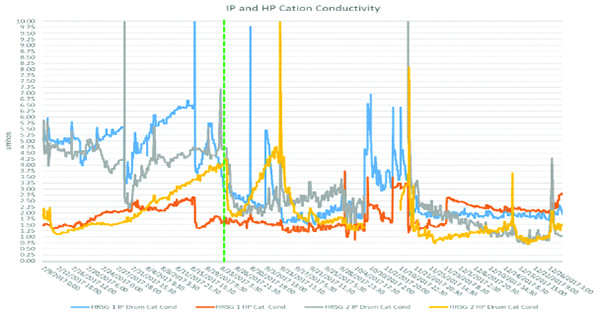 |
| 1. Intermediate-pressure (IP) and high-pressure (HP) drum cation conductivity. Courtesy: ChemTreat Inc. |
 |
| 2. Drum pH before and after modifications made on August 23, 2017. Courtesy: ChemTreat Inc. |
Amine Discussion
It is common for newly commissioned combined cycle plants to use straight ammonia for cycle chemistry pH control, owing to ammonia’s zero contribution to cation conductivity. Conversely, organic amines, such as ETA, are typically prohibited due to their breakdown into organic acids, which can contribute to cation conductivity.
Due to ammonia’s volatility at low pressures, it does not tend to remain in the LP drum, which in turn causes a depressed pH (less than 9.4) relative to the IP and HP drums—a significant drawback to an ammonia-only program. Four environmental factors have been identified for FAC to occur: a pH less than 9.4, a temperature range of 250F to 450F, carbon-steel construction, and operation in a reducing atmosphere (0 ppb dissolved oxygen). HRSG LP circuits, as well as IP and HP economizer inlets, experience temperatures in the 295F to 305F range, are constructed of carbon steel, and are frequently operated in a reducing environment—three out of the four factors. When coupled with an ammonia-only program, the probability of FAC occurring is high.
 |
| 3. Single-phase flow-accelerated corrosion (FAC) on pipe interior prior to feedwater pump suction. Courtesy: Electric Power Research Institute (EPRI) |
 |
| 4. Two-phase FAC in deaerator interior. Courtesy: EPRI |
Figures 3 and 4 show an example of both single-phase and two-phase FAC. It can appear smooth, rippled, black, black/red, or shiny silver, depending on environmental conditions. Flow-related corrosion has also been observed in areas where initial condensation occurs, such as at the back end of an LP turbine or inside an ACC. This type of FAC is occurring at much lower temperatures (from 90F to 130F) and typically appears as a smooth, shiny, silver surface (Figures 5 and 6).
 |
| 5. FAC inside an air-cooled condenser. Courtesy: John Monahon / Panda Liberty |
 |
| 6. FAC inside a water-cooled condenser. Courtesy: ChemTreat Inc. |
To minimize all forms of FAC, research and experience has led many plants to convert from an ammonia-only program to a blended-amine program, specifically ammonia/ETA, once commissioning is complete. What makes a blended-amine program better at reducing flow-related corrosion?
The answer has to do with amine volatility, that is, the way an amine moves throughout a HRSG. Every amine has a vapor-to-liquid ratio (V:L), representing the distribution of a particular amine in a steam/liquid mixture. For instance, an amine with a high V:L ratio prefers to go with the vapor phase relative to the liquid phase, while an amine with a low V:L ratio prefers to remain in the liquid phase. To further complicate the matter, V:L ratios change with pressure.
When feeding an ammonia-only program, it is difficult to elevate LP drum pH due to ammonia’s high V:L ratio at the lower pressure. Conversely, in the HP drum, the percent of ammonia in the liquid phase increases significantly because ammonia’s V:L ratio is much lower; thus, the HP drum pH is much higher, relatively speaking. Recall, experience has shown that to minimize FAC, it is necessary to maintain a pH of, at the very least, 9.4 in the condensate, feedwater, and LP drum. At these circuits’ operating pressures, it takes relatively more ammonia to achieve the target pH because ammonia does not remain in the liquid phase.
This is where organic amines come into the picture. Relative to ammonia, ETA has a low V:L ratio at all HRSG operating pressures. For comparison, ammonia is 10 times more volatile than ETA at LP operating temperatures and pressures. This means that ETA tends to stay in the liquid phase throughout the HRSG cycle chemistry to include the condensate, feedwater, LP drum, and liquid phase of any initial condensation areas, that is, water-cooled condenser structures, ACCs, and feedwater drain coolers.
It is ETA’s ability to maintain a consistent pH in the liquid phase of the cycle chemistry that makes it valuable when combating all forms of FAC. ETA does a much better job at buffering the low-temperature/low-pressure areas to a pH well above 9.4, thus eliminating one of the four factors necessary for FAC. Due to its low volatility, the pH of any liquid phase developed during the initial condensation (such as the back end of the LP turbine) is considerably higher than with the ammonia-only treatment. As liquid begins to form in the phase-transition zone (the area near the last three rows of blades), using an ammonia-only program means that most of the amine present is going to go into the vapor phase, thus leaving the liquid phase with very little pH buffering. Hence the reason initial condensation has been found to have a very low pH. This high-velocity, low-pH, high-purity liquid is sent to the water cooled-condenser or ACC unit, where, upon contact, it causes dissolution of the carbon-steel components. When using a blended amine, the ETA stays with the initial condensation, further buffering the pH and making the water less aggressive.
Extreme differences in V:L ratios mean the answer is not ETA or ammonia alone, it is a blend. Electric Power Research Institute studies and practical experience have shown the proper blend provides more basicity at typical utility operating temperatures and pressures, provides more consistent distribution throughout a multi-pressure HRSG, improves pH buffering of the LP and feedwater circuits, improves pH buffering in any area where there is initial condensation, and offers better protection from two-phase FAC because the ammonia buffers the vapor phase while the ETA buffers the liquid phase.
The biggest problem with organic amines is the degradation byproducts—acetic acid (which breaks down further into formate and carbon dioxide), formic acid, and ammonia. These breakdown products contribute to cation conductivity, which is the main concern in practical applications. The rate of amine degradation is very dependent on the maximum temperature to which the amine is subjected. In many HRSGs, superheat steam temperatures can reach 1,000F to 1,050F; temperatures that have resulted in organic amine degradation.
Blended amines have proven to be very effective tools in maintaining HRSG cycle chemistry. However, due to the nature of the chemistry, the organic portion of these blends ultimately degrades to acetic acid, formic acid, propionic acid, and CO2. It is how and where these acids accumulate that is important to anyone tasked with maintaining cycle chemistry.
Organic Acid Movement and Accumulation
As organic amines are subjected to superheat temperatures, they degrade into acids, with acetic and formic acid being the dominant species. These two acids move and accumulate differently relative to each other throughout the HRSG. In practical application, the author has found that these acids accumulate to a greater extent in the IP drum than in the HP drum in a feed-forward LP drum configuration. In a SALP drum configuration, acids accumulate in the LP drum to a greater extent than in the IP and HP drums.
As organic acids are generated in the superheat circuits, they travel to the steam turbine and then the condenser, with a portion of the acids being removed through the condenser’s air-removal section. The remaining acids are ionized in the condensate and ultimately end up in the HRSG drums. The acids then either accumulate in the drums or volatilize, making the loop again with some acids being removed in the air-removal section. Where these acids accumulate is based on several factors, with pressure, temperature, and pH being the focus of this article.
All three affect the volatility and degradation of organic acids. Volatility is affected by pH because it is the pH that dictates whether the volatile acid or the ionized salt is formed. For instance, the pKa values for acetic and formic acids are 4.76 and 3.76 at 77F (25C), respectively, but are significantly higher at 662F (35OC), 7.97 and 5.23, respectively. This means that at their respective pKa, 50% is acid and 50% is salt. The higher the pH the more the salt dominates; the lower the pH the more the volatile acid dominates. As it pertains to IP and HP drums, a drum pH (25C) of 9.5 correlates to a pH(t) of approximately 6.15 to 6.25. Meaning, acetate tends to volatilize in the HP drum and ionize (accumulate) in the IP. Theoretically, higher concentrations of acetate should be found in the IP drum relative to the HP drum, which has been confirmed with real-world cycle chemistry analyses.
Given formate’s pKa of 5.23 at 662F, it would be expected that a large portion would ionize and remain in the liquid phase, leading to high concentrations in drum water analyses. However, relatively little formate is found in IP drum samples, and even less in HP drum samples, all due to formate’s lack of thermal stability. Formate has shown poor thermal stability at temperatures at or above those seen in IP and HP drums. Formate shows much higher volatility than acetic acid, particularly at higher temperatures and pressures, which would explain why higher concentrations of formate are seen in HP steam samples relative to HP drum samples. External research has confirmed acetate dominates relative to formate production as temperatures and pressures increase. Therefore, the result of feeding organic amines or blended amines tends to be acid accumulation in IP drums. In severe cases, a slight to significant pH depression accompanies this accumulation.
But who cares? What is the point of this discussion? Are these byproducts causing any real adverse effects within HRSG cycle chemistry that warrant consideration and action? It is well-researched that these byproducts increase cation conductivity to above the standard upper limit of 0.2 µmhos. But is that really a problem? In the author’s experience, the answer is yes. Granted, examples of turbine failures directly attributed to organic acids are hard to find, but these acids do in fact cause operational issues.
The key point here is that, without a doubt, these acids will be generated when feeding an organic amine. Condensate cation conductivities in ACC units routinely increase above 1.0µmhos, depending on pH target and neutralizing amine/blend used. This elevated cation conductivity may not be an issue from a technical standpoint, but it is an issue from a human standpoint. When people, who possibly have little HRSG/ACC operating experience, see these cation conductivities, cycle chemistry discussions can get tense. Depending on one’s experience and knowledge base, a decision may be made to go to an ammonia-only program, which has other problems as previously discussed.
Furthermore, these acids readily accumulate in the SALP and IP drums of HRSGs. They accumulate to a lesser extent in HP drums, but in a HRSG’s LP and IP drums, the organics accumulate readily and sometimes at significant concentrations. It has been the author’s experience that the amount of acids accumulated can exceed 30,000 ppb, which will depress drum pH. This leads to the feed of more amine, contributing more acids—then the vicious cycle begins. This phenomenon mirrors glycol contamination in a HRSG because the same chemical reactions take place.
This is not to argue the benefits previously discussed but it is important that plant personnel are well-educated on the pros, cons, and what to expect when feeding this chemistry.
Relating Science to the Real World
Having a better understanding of neutralizing amines and their pros and cons, how does it pertain to the case study? Recall, prior to the mechanical modifications that took place on August 23, the pH control across the triple-pressure HRSG was very consistent and stable between 9.5 and 9.7. After modifications, pH control was very inconsistent, with the HP drum pH rising to nearly 10.0, and the IP and LP drum pH dropping below 9.0. So, what is happening, why is it happening, and most importantly, what can be done to correct the situation?
ETA breaks down into organic acids as it spends time at superheat temperatures. This is the first clue into what is taking place in the case study. Consider this, a blended amine is fed to the condensate pump discharge. That amine travels to the drums, where a portion stays with the drum water and a portion volatilizes to the steam. Depending on conditions, a percentage of the volatile amine breaks down into organic acids. As the steam performs work in the turbine, it is exhausted to the ACC.
Inside the ACC, some volatile amine and organic acids are removed through the air-removal system, while some travel back around via the condensate. If the amine is ammonia-only, there is no degradation, and the percent amine recycle should be very high. If the amine is ETA or a blended ammonia/ETA, a portion will degrade at each cycle within the HRSG. With the recycling of ammonia, the amount of fresh blended-amine fed to the system continues to be reduced until the system is essentially an ammonia-only system. Based on the science behind ammonia volatility, this explains why the pH started to spread, with the HP pH rising and the pH in the LP and IP decreasing.
The loss of ETA is also confirmed through cation conductivity readings. After August 23, the drum cation conductivities decrease, with the IP drum decreasing rapidly. What this indicates is that less organic acids are accumulating, which makes sense with the reduction in ETA being fed to the system. Herein lies the Catch-22. Feeding a blended-amine at the necessary dosage to minimize FAC in an ACC would appear to lead to elevated cation conductivities and high amine usage rates. While feeding an ammonia-only program, and recycling that amine through the modifications discussed, allowed for the significant reduction in amine feed, but set the plant up for FAC conditions.
Treatment Options
The author has struggled in assisting ACC plants with balancing pH control and cation conductivity throughout HRSGs. Personal experience has shown that when maintaining a pH of 9.6 to 10.0 with a blended amine, the cation conductivity is rarely below 1.0µmhos. However, using an ammonia-only program, the LP drum, LP generating banks, and IP/HP economizers are at serious risk for FAC given the inevitable low pH of these circuits.
Adding to the complication of choosing between ammonia-only and blended amines, there is the question of how these chemistries should be fed. The case study provided shows very clearly that mechanical modifications can optimize amine usage. But at what expense? In practical experience, the author has identified a few options for selecting and feeding neutralizing amines to HRSGs with ACC units.
Feed Ammonia-Only to Target 9.8 pH in the LP Drum. This would require approximately 25 ppm of 19% ammonia to achieve a pH of 9.8. If the vacuum pump drain is recycled, ammonia loss should be minimal and should lower the feed rate. Even though some ammonia will be lost in the air phase of the vacuum pump, it should not be significant. When recycling the vacuum pump drain, the case study shows that when HP drum pH is above 9.9, the LP drum pH is at 9.2 due to ammonia volatizing in the steam phase. The upside to an ammonia-only program is that there are no organics present, so there will be no cation conductivity addition. As a side note, it is important to understand sample panel cation columns will exhaust more rapidly due to the exchange of ammonium ions with the column’s hydrogen.
Feed Ammonia/ETA Blend to Target 9.6 pH in the LP Drum, without Recycling the Vacuum Pump Drain. This has proven to require a significant amount of amine given the losses. The blend does provide a much better pH profile across the HRSG drums, but at what cost. Could losses be controlled by optimizing vacuum pump operation? In other words, does artificially reducing the vacuum pump capacity (creating air in-leakage ahead of the pump or increasing seal water temperature) minimize the draw of amine out of the system? If the vacuum pump drain is to be recycled, there is no need to feed this blend. The data indicates the ETA degrades over time and the system is left essentially an ammonia-only program. This is confirmed by not only the pH profile, but the reduction in cation conductivity throughout the system because the ammonia is satisfying the specific conductivity setpoint.
Feed Blended Amine to the IP/LP Turbine Crossover Piping. This would mean the ACC sees an elevated pH to minimize FAC prior to any degradation of the organic portion of the amine. A condensate pH target of 9.6 to 10.0 would still be necessary to minimize FAC in the LP circuits, and IP/HP economizers. If the amine were fed to the IP/LP turbine crossover it could allow for a reduced feed rate of the blended amine. Because the amine is not subjected to the elevated final superheat steam temperatures initially, very little degradation would occur. It should be noted that amine will eventually cycle to the drums and superheat steam, which will cause a portion of the amine to convert to organic acids. So, the production of organic acids is going to occur, but the ACC is going to see a larger amount of intact amine; therefore, pH control within the ACC will be much more consistent.
Regardless of the chosen amine and feed point, HRSG complexities, particularly those with ACCs, make it difficult for chemistry managers to adequately protect these systems. The key is monitoring. Careful and accurate monitoring of the system (cation conductivity, pH, and iron) is the only way to determine the effectiveness of a cycle chemistry program. ■
—Kevin Boudreaux (Kevinb@chemtreat.com) is an industry technical consultant with ChemTreat Inc. Special thanks to John Monahon and the plant personnel at Panda Liberty, and Mark McIntyre of ChemTreat for all their help and support in the development of this article.