Researchers at Oregon State University (OSU) have developed a method to manufacture nanoporous graphene for use in supercapacitors from atmospheric carbon dioxide (CO2). Graphene is a form of carbon that is essentially a one-atom-thick layer of graphite, in which the carbon atoms are arranged in a hexagonal lattice. Because of its virtually two-dimensional character, it has a variety of fascinating chemical and physical properties. Graphene is 100 times stronger than steel and is an excellent conductor of heat and electricity.
Nanoporous graphene is graphene in which nanopores have been created in the lattice (Figure 8). It has a very high specific surface area, about 1,900 square meters per gram. This gives it an electrical conductivity at least 10 times higher than the activated carbon currently used to make commercial supercapacitors.
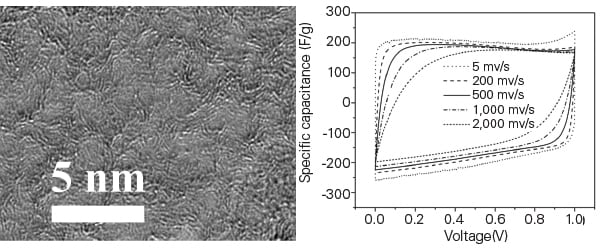 |
| 8. Small pores, big potential. A method for manufacturing nanoporous graphene holds the potential for creating vastly more powerful supercapacitors. Courtesy: Oregon State University |
However, the method developed at OSU to create nanoporous graphene is faster, less expensive, and has less environmental impact than previous methods such as chemical etching, which often use toxic materials. Rather than etching graphene, the OSU method uses a mixture of magnesium and zinc metals that are heated to high temperature in a flow of carbon dioxide. This produces a controlled reaction that converts the elements into their metal oxides and nanoporous graphene.
Because of its simplicity and low cost, OSU researchers believe the method has good potential to be scaled up for commercial manufacture. Supercapacitors with nanoporous graphene electrodes could potentially have far higher storage capacity than current designs using activated carbon.
—Thomas W. Overton, JD