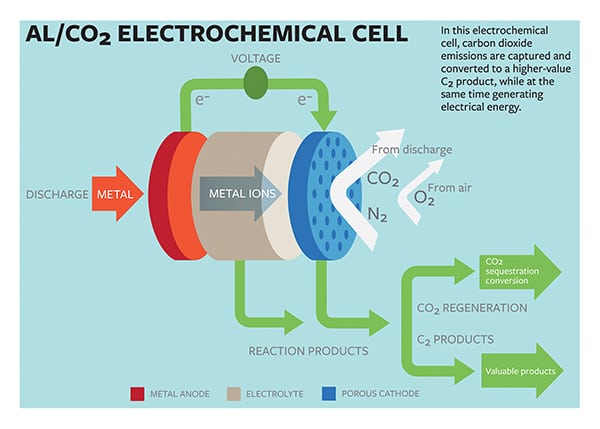
Researchers from Cornell University have developed an oxygen-assisted aluminum/carbon dioxide power cell that uses electrochemical reactions to capture carbon dioxide (CO2) and produce power in the process.
The cell developed by Lynden Archer, the James A. Friend Family Distinguished Professor of Engineering, and doctoral student Wajdi Al Sadat would use aluminum as the anode and mixed streams of CO2 and oxygen as the active ingredients of the cathode (Figure 5). The electrochemical reactions between the anode and the cathode would sequester the greenhouse gas into carbon-rich compounds while also producing electricity and a valuable oxalate as a byproduct, the university explained in a press release.
The findings in the study (published on July 20 in the journal Science Advances and funded by a grant from the Saudi Arabian King Abdullah University of Science and Technology Global Research Partnership program) represent a possible paradigm shift, Archer said. Most current carbon capture models capture the carbon in fluids or solids. These are then heated or depressurized to release the CO2, but the concentrated gas must be compressed and transported for reuse or storage.
“One of the roadblocks to adopting current carbon dioxide capture technology in electric power plants is that the regeneration of the fluids used for capturing carbon dioxide utilize as much as 25% of the energy output of the plant,” Archer said. “This seriously limits commercial viability of such technology.”
The electrochemical cell generated 13 ampere-hours per gram of porous carbon (as the cathode) at a discharge potential of around 1.4 V. “The energy produced by the cell is comparable to that produced by the highest energy-density battery systems,” suggested the researchers. It also generates superoxide intermediates, which are formed when the dioxide is reduced at the cathode. The superoxide reacts with the CO2, a normally inert compound, forming carbon-carbon oxalate, which is widely used in many industries, including pharmaceutical, fiber, and metal smelting.
“A process able to convert carbon dioxide into a more reactive molecule such as an oxalate that contains two carbons opens up a cascade of reaction processes that can be used to synthesize a variety of products,” Archer said, noting that the configuration of the electrochemical cell will be dependent on the product one chooses to make from the oxalate.
However, the design has a drawback, the researchers noted. Its electrolyte is “extremely sensitive to water.” Work continues to address the performance to electrochemical systems and the use of electrolytes that are less water-sensitive.
—Sonal Patel, associate editor