Forgotten water: Stator cooling water chemistry
Even utilities that are very diligent about treating and monitoring their boiler water, demineralized water makeup, and cooling water may know little about treating one of the most critical water systems in the plant: stator cooling water.
Stator cooling water is contained in a closed loop system that cools the copper stator bars in water-cooled generators. The holes though which the water flows are narrow. Unimpeded flow through all stator bar openings is critical to operation of the generator. Overheating of stator bars can result in reduced generating capacity or even catastrophic failure of the generator.
Stator cooling systems contain only deionized water. The cooling loop removes heat from the stator coolers and conveys it away through heat exchangers. The water is continuously passed through a mixed bed polisher that removes any soluble ionic contaminants that enter the water. The stator ion exchange resins often also act as a filter for particulates in the water, though some systems have a separate filter. The ion exchange resin will eventually become exhausted, but in many systems, it is common for the pressure differential across the resin bed (created by accumulated particulates) to require that the resins be replaced before the ion exchange capacity is reached.
Copper contamination
The stator cooling water system’s heat transfer surfaces are typically copper, though some are stainless steel. The chemistry of copper in oxidizing and reducing conditions has been the subject of a great deal of recent research. One area of intense focus has been copper corrosion in feedwater systems and corrosion product transport into the boiler and on to the HP turbine. Some of the general metallurgical principles learned in studying copper in feedwater systems can also be applied to stator cooling systems. As with copper in feedwater systems, we know that dissolved oxygen and pH play a major role in determining the corrosion product formation rate and transportation rate through the stator cooling system.
It is important to remember that the major cause of problems in stator cooling systems has not been corrosion per se but, rather, deposit accumulation in critical areas. These deposits are copper oxides released from one area of the stator coolers and deposited in another. The amount of dissolved oxygen in the system, and particularly variations in that oxygen concentration, determines when copper oxides are released.
Copper forms cuprous oxide (Cu2O) under reducing (low-oxygen) conditions and cupric oxide (CuO) when dissolved oxygen is high. Either of these oxides can be stable and create a passive oxide layer on the channels in the stator bars. A slightly alkaline pH increases the stability of the oxide layer.
Study your options
The recommended treatment regimes for stator cooling water can be categorized by their levels of dissolved oxygen and pH. Of the four options illustrated in Figure 1, three are generally recommended by treatment experts. Each of the recommended options can be found in operating power plants, and each has pros and cons that must be balanced against the particular needs of a plant and its equipment operating history.
|
1. Three real options. Only three of the four possible stator cooling water treatment options are viable. Source: M&M Engineering
|
Low-O2, neutral pH option. A thin layer of passive cuprous oxide forms in a low–dissolved oxygen and neutral pH regime. This treatment option is found in about 50% of the stator cooling systems in the power industry. The water is fully oxygenated when the system is first filled. As the water circulates, it reacts with the copper in the system, the oxygen is consumed, and the dissolved oxygen gradually approaches zero. The dissolved oxygen is likely to remain at <10 ppb as long as no water is added to the system. Neither oxygen scavengers nor reducing agents are commonly added to stator cooling systems. The trick in this treatment regime is keeping dissolved oxygen out.
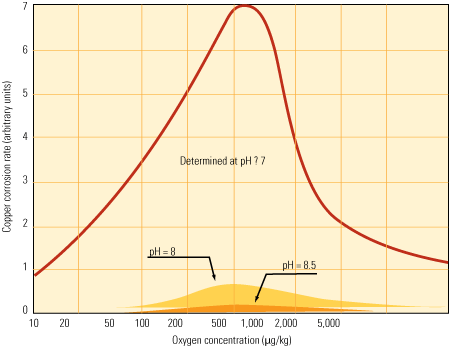
The effect of transient oxygen spikes is most profound on low–dissolved oxygen and neutral pH systems. The corrosion or oxidation rate of copper in a system containing 400 ppb of dissolved oxygen is more than five times its rate at low levels of oxygen under neutral conditions.
Every time makeup water is added to the stator system, it receives a little shot of dissolved oxygen. If, due to leaks in the stator cooling water system, makeup rates are significant, the stator cooling water can swing back and forth between low- and high-oxygen conditions. These transient conditions release oxides into the system.
Air ingress can also cause significant increases in the system’s dissolved oxygen. Putting a nitrogen cap on the stator cooling water head tank can minimize air in-leakage.
Carbon dioxide can enter the system via the makeup water or along with the air. As carbon dioxide is absorbed into the water, it drops the pH to acidic levels, increasing the corrosion rate of copper. Carbon dioxide can form bicarbonate and carbonate in the water and exhaust the mixed bed polisher. If the polisher is not changed when it is exhausted, the released carbonate can form insoluble copper carbonate in the stator.
To prevent dissolved oxygen contamination resulting from additions of makeup water, some utilities have turned to oxygen removal systems. The Tarong Power Station in Queensland, Australia, uses a series of three gas transfer membranes to remove dissolved oxygen from any makeup water added to its stator cooling system. The system, which uses only nitrogen purge gas to sweep out the oxygen that permeates the membrane, is capable of reducing the dissolved oxygen of the makeup water to about 3 ppb.
Low-O2, higher pH option. Increasing the pH of the stator water to 8–9 significantly reduces the corrosive response during oxygen transitions (Figure 2). The most direct method for increasing pH is to add controlled amounts of sodium hydroxide to the water. Initially, the sodium will be exchanged with hydrogen on the cation resin of the mixed bed polisher, neutralizing the caustic. If caustic continues to be added, eventually, sodium leakage from the resins will allow the water to maintain an alkaline pH.
|
2. Oxygen therapy. The effect of pH on copper corrosion rates is significant as oxygen levels increase. Source: Journal of Power Plant Chemistry
|
Another treatment method is to add a sodium exchange polisher on a side stream and control the amount of water that passes through the sodium exchanger to achieve the desired pH. Some plants have even replaced the mixed bed polisher with all strong base anion resins. However, this polisher will no longer remove soluble copper. Raising the pH also makes it easier to measure the pH of water in the system.
During shutdown, and particularly during a major turbine outage, stator water can become oxygenated. In a number of cases, deterioration of the stator cooling system occurred shortly after the unit came back on-line from an extended outage.
High-O2, neutral pH option. The other treatment alternative is to maintain a high–dissolved oxygen level in the cooling water at all times. It is estimated that 40% of water-based stator cooling systems operate with high–dissolved oxygen and neutral water chemistry. In this treatment regime, CuO is formed on the copper. It will tightly adhere to the surface and create a passive layer on the metal. This layer tends to be thicker than the Cu2O formed under low-oxygen conditions.
Because the dissolved oxygen will be depleted by its reaction with copper, at least initially, you may need to add air to the system to maintain sufficient dissolved oxygen in the system.
This chemical treatment is impervious to additions of dissolved oxygen in the feedwater when it is operating continuously under high (>2 ppm) levels of dissolved oxygen. However, it may still be susceptible to low-pH corrosion from carbon dioxide and carbonates if these are not removed by the mixed bed polisher.
If there is a hydrogen leak into the stator cooling water system, the hydrogen can replace the dissolved oxygen and create low–dissolved oxygen transients in the system, causing oxides to be released.
High-O2, high-pH option. Operating with high dissolved oxygen and an elevated pH is not recommended because it increases the likelihood of clip corrosion.
Monitoring stator water
Monitoring the health of stator water systems is more about looking at a variety of related temperatures and pressures than collecting grab samples and running them for pH or dissolved oxygen. Water temperature is one example: An increase in stator cooling water temperature puts the cooling water system at higher risk for plugging.
Monitoring the makeup water usage in a stator cooling system is also important. If the system is operating under a low–dissolved oxygen regime, an increase in makeup water may signal a potential problem from oxide accumulations. Monitoring gas flow rates from the stator cooling water head tank is one way to detect hydrogen leaks that can deplete dissolved oxygen in the water, if you are using the high-oxygen regime.
Conductivity of the water and pressure drop across the polisher are critical monitoring parameters and should be monitored continuously with carefully selected setpoints. The frequency with which the filter needs to be changed, due to particulate plugging, is an indication of corrosive conditions in the system. The resins themselves can be tested to determine the amount and nature of the copper that is being transported through the system.
Generators should also be equipped with flow and pressure differential metering of the cooling water system across the generator. These parameters can also provide an indication of fouling if they are monitored regularly and trended.
On-line monitoring for dissolved oxygen is recommended if the plant will be operating under a low-oxygen regime. Grab sampling is not usually recommended for these systems due to the amount of water required to flush out sample lines before one can be sure of getting an accurate sample. This water is then replaced with oxygenated water, thus changing the analysis.
—David G. Daniels ([email protected]) is a principal of M&M Engineering and a contributing editor to POWER.