Determining Carbon Capture and Sequestration’s Water Demands
The U.S. Department of Energy’s National Energy Technology Laboratory is pursuing a new integrated energy-water R&D program that addresses water management issues relative to coal-fired power generation that takes into account the major impacts of CCS on water use. The goal of this research is to promote more efficient use of water in power plant operations and increase the availability of heretofore unusable waters for power plant use. Those practices can mitigate the impacts of CCS on power plant water use and allow for continued development of energy resources.
The production of energy requires a reliable, abundant, and predictable source of freshwater — a resource that is limited in many parts of the U.S. As we experience increasing demand for energy and water and growing limitations on supply, these resources must be managed together to maintain reliable energy and water supplies that will enable full use of the nation’s energy reserves. This may become a larger issue in a carbon-constrained world, given that current carbon capture technologies may result in increased water usage. Recent studies conducted by the U.S. Department of Energy’s National Energy Technology Laboratory (DOE/NETL) indicate that deployment of carbon dioxide (CO2) capture systems could significantly increase power plant water usage.
A key objective of DOE/NETL research and development (R&D) activities is to mitigate any impact of carbon capture on water resources. DOE/NETL is addressing this issue under two key components of its Existing Plants, Emissions, and Capture (EPEC) Program:
-
The development of advanced CO2 capture technologies that require less freshwater.
-
The development of advanced water management technologies that reduce freshwater usage throughout the power plant and that encourage the use of waters that heretofore were thought to be unacceptable for use.
Water Usage in Electricity Generation
Thermoelectric power generation requires significant quantities of water. For example, a 500-MW coal-fired power plant uses more than 12 million gallons of water per hour. The largest demand for this water is process cooling (the conversion of steam to liquid water in a condenser).
The two commonly used metrics to measure power plant water use are withdrawal and consumption. "Consumption" is used to describe the loss of withdrawn water — typically through evaporation into the air — that is not returned to the source. Water "use" or "withdrawal" describes drawing on water sources, typically freshwater, for process use and then returning it to the original source. So even though the plant described above may use 12 million gallons per hour, depending on the type of cooling system used, water consumption will be a very small fraction of that.
In thermoelectric power plants, there are basically two types of cooling water system designs: once-through (open loop) and recirculating (closed loop). Dry cooling systems do exist, but they are not widely used because of their negative impact on power plant efficiency and their high first cost. In once-through systems the cooling water is withdrawn from a local water body and passed through the condenser, where heat from the steam is transferred to the cooling water. The used warm cooling water is subsequently discharged back to the same water body.
The most common type of recirculating or closed-loop system uses wet cooling towers to cool condenser water. In these systems the warm cooling water is typically pumped from the condenser to a cooling tower, where the heat is dissipated directly to ambient air by evaporation of a fraction of the water and by heating the air. The remaining cooling water is then recycled back to the condenser. Because of evaporative losses, a portion of the cooling water needs to be periodically discharged from the system — known as blowdown — to prevent the buildup of minerals and sediment in the water that could adversely affect performance. For a wet recirculating system, only makeup water needs to be withdrawn from the local water body to replace water lost through evaporation and blowdown.
Withdrawal and Consumption
The U.S. Geological Survey (USGS) estimated that thermoelectric generation accounted for approximately 41% of freshwater withdrawals, ranking slightly ahead of agricultural irrigation as the largest source of freshwater withdrawals in the U.S. in 2005. However, thermoelectric water consumption accounted for only 3% of total U.S. freshwater consumption in 1995 (Figure 1). A recent DOE/NETL study estimated that in 2005 total U.S. freshwater withdrawals for thermoelectric power generation amounted to approximately 146 billion gallon per day (bgd), while freshwater consumption was 3.7 bgd. When evaluated in terms of the two types of cooling water system designs described above, once-through systems are thought to have high withdrawal but low consumption, whereas plants equipped with wet recirculating systems have relatively low water withdrawal but high water consumption.
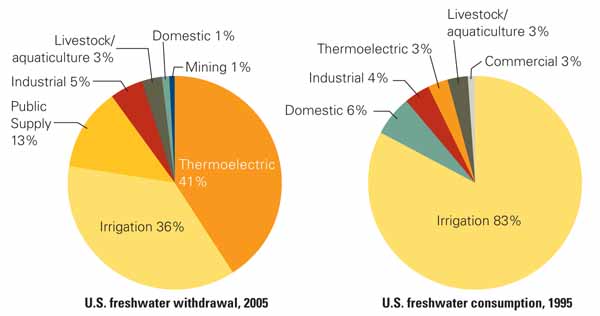
1. Consumption vs. withdrawal. These charts present statistics related to U.S. freshwater consumption and withdrawal as estimated by the USGS. NETL withdrawal data are for 2005 and consumption data are for 1995. Source: U.S. Department of Energy’s National Energy Technology Laboratory
One of the key considerations in evaluating the relationship of energy and water is the increasing demand for both commodities. Thermoelectric generating capacity is expected to increase by nearly 11% between 2005 and 2030, based on the Energy Information Administration’s (EIA) Annual Energy Outlook 2009 (AEO 2009) projections. Depending upon the assumptions invoked, water withdrawal to support electricity generation is expected to stay the same or decline slightly over the same time period. However, water consumption is expected to increase by anywhere from 28% to nearly 50% on a national basis.
Withdrawal is expected to remain the same or decrease because plants likely to be retired between 2005 and 2030 are older facilities that are more likely to employ high-withdrawal, once-through cooling. New facilities that will be built over that time period are likely to employ lower-withdrawal but high-consumption wet recirculating cooling systems. These projections are based on a business-as-usual approach and do not reflect responses to potential energy/climate legislation being proposed by Congress.
The Impact of CO2 Capture on Water Use
In existing and future fossil fuel – fired power plants, CO2 capture technologies would offer a mechanism for reducing CO2 emission levels. In fact, if coal is to remain an important fuel for power production in the U.S. under any future climate/energy legislation, carbon capture and sequestration (CCS) will be essential.
However, carbon capture comes at an additional cost in terms of both money and resources needed to manufacture, install, and operate the advanced technologies. The components of any power plant require a portion of the electricity generated by the plant in order to operate, in addition to large quantities of freshwater for cooling. The use of currently available carbon capture techniques adds additional demands for electricity and water on top of this baseline resource load. The additional water required for a power plant with CO2 capture technology is largely due to the additional cooling water requirements used during capture and compression.
Carbon capture technologies can be divided into three general categories based on the types of systems to which they are applied: postcombustion, precombustion, and oxy-combustion. Postcombustion techniques are generally used for conventional pulverized coal – fired (PC) power generation, but they can also be applied to natural gas combustion turbines. Precombustion technology can be applied in situations in which fossil fuel is gasified, producing a CO2 waste stream that can be readily captured prior to combustion of the gasified fuel. Oxy-combustion is a process in which coal combustion occurs with an oxidant stream made up of pure oxygen or a mixture of oxygen and recycled CO2, resulting in an outlet stream that is essentially only CO2 and water vapor. This article focuses only on postcombustion and precombustion processes.
Postcombustion CO2 Capture
Amine-based wet scrubbing (such as monoethanolamine — MEA) has been used commercially for the removal of acid gases (CO2 and H2S) from various processes such as natural gas purification and food-grade CO2 production for more than 70 years. Although they are not currently deployed at scale, the existing wet-scrubbing technologies could be scaled-up and fitted onto a new or existing power plant, as shown in Figure 2.

2. Trapping carbon. This diagram depicts postcombustion CO2 capture for a pulverized coal power plant. Source: U.S. Department of Energy’s National Energy Technology Laboratory
After air pollutant clean-up (such as the removal of NOx, SOx, particulate matter, and Hg), the combustion flue gas enters an absorber reactor and flows countercurrently to a CO2 -lean amine solution, where CO2 is removed from the flue gas. The treated flue gas (essentially nitrogen) is discharged to the atmosphere, and the CO2 -rich solution is pumped to a stripping reactor for regeneration. In the stripping column, the CO2 -rich amine solution is heated in order to reverse the reactions and ultimately regenerate the amine solution. A reboiler provides the heat for regeneration using steam extracted from the turbine cycle. Consequently, CO2 is released, producing a concentrated stream that exits the stripper and is then cooled and dried in preparation for compression, transport, and storage. The CO2 -lean solution leaving the stripper is cooled and returned to the absorber for reuse.
Precombustion CO2 Capture
Precombustion CO2 capture systems are designed to separate CO2 from high-pressure synthesis gas (syngas) produced in integrated gasification combined-cycle (IGCC) power plants. By carefully controlling the amount of oxygen introduced to the gasifier, only a fraction of the fuel burns, providing the heat necessary to decompose the coal and produce syngas, a mixture of hydrogen (H2) and carbon monoxide (CO), along with minor amounts of other gaseous constituents.
To enable precombustion capture, the syngas is further processed in a water-gas-shift (WGS) reactor, which converts CO into CO2 while producing additional H2, thus increasing the CO2 and H2 concentrations. Water, in the form of steam, is needed for the WGS reaction to take place: CO + H2O = CO2 + H2. A physical-based acid gas removal system, such as Selexol, can then be used to separate the CO2 from the H2. The CO2 is dried and compressed in a multi-stage, intercooled compressor to supercritical conditions for pipeline transport. The majority of water used in the compression process goes toward cooling.
Water Requirements for Pre- and Postcombustion CO2 Capture
A recent DOE/NETL study established baseline cost and performance data for a variety of power plant configurations both with and without carbon capture. Subcritical pulverized coal, supercritical PC, and IGCC configurations were evaluated for a variety of factors, including water withdrawal and consumption. The evaluation results in terms of water use are presented in Figure 3.
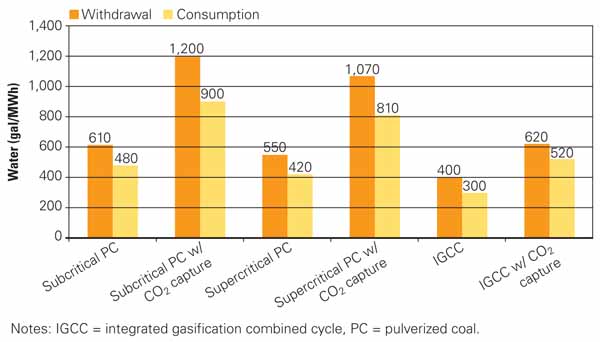
3. Thirsty plants. This chart shows the relative water usage for new pulverized coal and integrated gasification combined-cycle power plants. Source: U.S. Department of Energy’s National Energy Technology Laboratory
For the subcritical PC configuration, water withdrawal increased from just over 600 gallons per megawatt-hour (gal/MWh) to nearly 1,200 gal/MWh, while consumption increased from less than 500 to around 900 gal/MWh.
For the supercritical PC configuration, water withdrawal increased from about 550 gal/MWh to around 1,050 gal/MWh, while consumption increased from just over 400 gal/MWh to just over 800 gal/MWh.
IGCC water withdrawals increased from around 400 gal/MWh without carbon capture to just over 600 gal/MWh with capture, while consumption increased from approximately 300 gal/MWh to about 500 gal/MWh.
The results show a significant increase in overall water use by subcritical and supercritical PC plants but a more modest increase for IGCC plants. One of the major reasons for this difference is that only one-third of the power generation of an IGCC plant is based on the steam turbine, which requires cooling water for steam condensation. The other two-thirds of IGCC power generation is from gas turbines, which don’t require cooling water. All of the configurations evaluated were assumed to use wet recirculating cooling, resulting in withdrawal and consumption estimates that are similar within a given configuration.
A subsequent study has evaluated five different scenarios for future freshwater withdrawal and consumption requirements for U.S. thermoelectric generation using the AEO 2009 projections for capacity additions and retirements. Results from this study indicate that carbon capture and sequestration will increase water withdrawal by 5% to 7% by 2030, but consumption will increase by 88% to 100% over the same time period.
NETL’s Carbon Capture Research Program
NETL’s EPEC program is conducting R&D on advanced CO2 capture technologies for new and existing PC power plants. Postcombustion carbon capture research focuses on four technology pathways: membranes, solvents, sorbents, and compression. One of the principal advantages of all the technologies being evaluated is that they have significant potential to capture CO2 with much lower parasitic load (versus current wet scrubbing), thereby decreasing the water usage impact of CO2 mitigation.
Gas Separation Using Membranes. This process occurs via physical or chemical interactions between the membrane and the gas being separated. Specifically, one component in the gas permeates the membrane faster than another.
In general, membrane processes offer several potential advantages: They operate passively, with no moving parts; they can be designed to withstand chemical contaminants (SOx and NOx); they are energy efficient, with low operating costs; and they are modular and have a small footprint. However, though membranes are best suited for separating CO2 in high-pressure applications, such as coal gasification, the EPEC program is focused on developing highly selective and permeable membrane systems designed specifically for CO2 separation from low-partial-pressure flue gas streams. These include hybrid systems that combine membranes with absorption liquids (such as amine solvent or enzymes), thin-film composite polymer membranes, the use of enzymes to catalyze the conversion of CO2 to carbonate, and other unique materials and configurations aimed at maximizing CO2 removal while minimizing costs and other impacts, including water use.
Solvent-Based CO2 Capture. This technology involves the chemical or physical sorption of CO2 from flue gas into a liquid carrier. Chemical absorption involves reversible chemical reactions between CO2 and an aqueous solution of an absorbent such as amine to form water soluble compounds.
Chemical solvents are able to capture high levels of CO2 from flue gas streams with a low CO2 partial pressure; however, there are significant cost and efficiency penalties during the regeneration step, which involves a temperature swing to reverse the chemical reactions. Physical adsorption is a bulk phenomenon where inorganic or organic liquids preferentially adsorb a gaseous species from the gas mixture. Although the regeneration of physical solvents is less energy-intensive than chemical-solvent regeneration, this technology is considered more practical for separating CO2 from high-pressure synthesis gas generated during coal gasification because CO2 solubility in physical solvents increases with partial pressure.
DOE/NETL is investigating advanced solvents that can be applied to high-volume, low-CO2 -concentration conditions that have lower regeneration heat duties, lower parasitic power demand for solvent recovery, and resistance to flue gas impurities.
Solid Sorbents. This technology takes advantage of the affinity of CO2 for certain solids. Materials being explored include sodium and potassium oxides, zeolites, carbonates, amine-enriched sorbents, and metal-organic frameworks.
As with solvent-based systems, a temperature swing facilitates sorbent regeneration following chemical and/or physical adsorption. However, a key attribute of CO2 sorbents is that less water is present than in solvent-based systems, thereby reducing the energy requirements (and thus water use) for sensible heating and stripping.
Research projects in this area address key technical challenges to sorbent-based systems, such as solids circulation, sorbent attrition, low chemical potential, heat transfer, reactive flue gas contaminants, and the parasitic power and sweep gas demand for sorbent regeneration.
The Relationship Between Geologic Carbon Sequestration and Water
After capture and compression, the CO2 can be stored through injection into various types of geologic formations such as depleted oil and gas fields, saline formations, and unminable coal seams. When injected into liquid-containing formations (oil and gas or saline), the gas permeates the rock and occupies part of the pore spaces while dissolving into the liquids. This can lead to changes in the physical and chemical properties of the liquids that can affect industry’s future ability to extract (such as with CO2 -enhanced oil recovery) and use (such as with cooling water) the liquids. At times, when CO2 is injected into these formations, produced water (the water extracted from the subsurface, typically during oil and gas production) will be generated.
The way in which the produced water is managed depends on the water quality characteristics. Produced waters from saline aquifers and depleted oil and gas reservoirs often have high total dissolved solids (TDS) and metals concentrations and must either be reinjected or treated to serve a beneficial use in a surface application. On the other hand, water produced in the process of enhanced coalbed methane (ECBM) extraction may even be potable or can be used for agriculture. The ECBM extraction process involves flooding unminable coal seams with CO2. The CO2 preferentially sorbs to the permeable media and is thus sequestered. Methane is released and can then be captured and put to a beneficial use. The water that is produced in this process is generally high-quality water.
NETL’s Power Plant Water Management R&D
In order to address issues related to water use in the generation of electricity, NETL is pursuing an aggressive R&D effort directed at developing technologies and approaches to better manage how coal-fired power plants use and impact freshwater resources. The overall goal is to reduce the amount of freshwater needed for power plant operations and to minimize potential impacts on water quality.
The research encompasses laboratory and bench-scale activities through pilot- and full-scale demonstrations and is built upon partnership and collaboration with industry, academia, technology developers, and other government organizations. The program is built around three specific areas of research: nontraditional sources of process and cooling water, innovative water reuse and recovery, and advanced cooling technology.
Nontraditional Sources of Process and Cooling Water Research. This research area seeks to develop innovative technologies to utilize lower-quality water for power plant cooling and related process needs. For example, one of the projects being conducted is titled "Reuse of Produced Water from CO2 Enhanced Oil Recovery, Coal-Bed Methane, and Mine Pool Water by Coal-Based Power Plants." The Illinois State Geological Survey is evaluating the feasibility of reusing three types of nontraditional water sources for cooling or process water for existing and planned coal-fired power plants in the Illinois Basin. The three types of nontraditional water sources are:
-
Produced water from CO2 -enhanced oil recovery (EOR).
-
Coal-bed methane recovery.
-
Active and abandoned underground coal mines.
Increasing public resistance to the withdrawal or consumption of freshwater for industrial purposes suggests that the use of nontraditional water sources in power plants may alleviate the expected increase in water demand. Power plant usage of water produced by CO2 -EOR may be an environmentally sustainable alternative for "closing the CO2 -water loop," as illustrated in Figure 4.
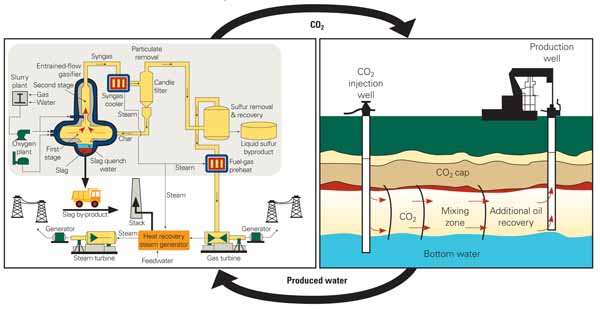
4. Closing the CO2-water loop. CO2 is produced in the generation of electricity and then it is injected into depleted oil and gas reservoirs to enhance oil recovery. Water produced in the process is treated and returned to the power plant for cooling and other purposes. Source: U.S. Department of Energy’s National Energy Technology Laboratory
Innovative Water Reuse and Recovery Research Area. This research area focuses on development of advanced technologies to recover and reuse water from such power plant processes as coal dewatering and flue gas dehumidification. One of the projects in this research area is called "Water Extraction from Coal-Fired Power Plant Flue Gas." Coal naturally contains water (3% to 60% by weight), and the combustion process releases additional water as hydrogen as the coal is reacted with oxygen. The amounts of water that can be recovered from flue gas are sufficient to substantially reduce the need for off-plant sources of water. Currently, there is no practiced method of extracting the water from power plant stack gas.
The University of North Dakota Energy and Environmental Research Center is conducting a project to capture water from flue gas in both PC and IGCC applications. This research uses liquid desiccant – based dehumidification technology to efficiently extract water from the power plant flue gas. It requires minimal heat rejection equipment, can function across the entire ambient range, and has minimal impacts on power plant efficiency.
The liquid desiccant – based system is placed at the exhaust end of a coal-fired power plant and utilizes low-grade heating and cooling sources available in the plant. The flue gas is cooled and then subjected to a liquid desiccant absorption process, which removes water from the flue gas. Once the water is removed from the flue gas, the desiccant is heated to remove the water, and the water vapor is condensed and can be used in other plant processes.
Advanced Cooling Technology. This component of the EPEC program is working to develop advanced wet, dry, and hybrid cooling systems. One of the projects under way in this research area is called "Air2Air Technology to Reduce Freshwater Evaporative Cooling Loss." For this project, SPX Cooling Technologies is investigating the use of an air-to-air heat exchanger above a wet cooling tower, as shown in Figure 5. It takes warm, humid air from the cooling tower and contacts it with cooler, dry air from outside to condense and recover a portion of the water that just evaporated from the cooling tower.
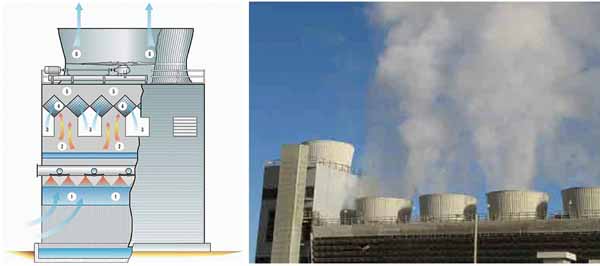
5. Air2Air cooling technology. The tower on the far left of the photo includes Air2Air technology. Note that the steam plume coming from the other towers is effectively eliminated in the Air2Air tower. Source: U.S. Department of Energy’s National Energy Technology Laboratory
This system has the potential to condense as much as 20% of the cooling water that would have been lost to evaporation. If this technology were applied to all wet recirculating cooling towers in the U.S., the water savings could reach 1.56 bgd. In addition, these units are very effective at steam plume abatement. Initial tests of the technology produced positive results, and now follow-up work is focused on analysis of improved geometries of airflow through the units as well as improved seals to enhance liquid capture.
Looking Ahead
The production of electricity, the deployment of CCS, and water use all raise a wide variety of societal issues, policy and regulatory debates, environmental questions, and technological challenges. Water in particular is emerging as a signiï¬cant factor in economic development activities. Given the extent to which CCS may increase water withdrawal and consumption, the impact of CCS on water resources must be considered and better understood in order to accommodate future energy and water needs.
Planning efforts must consider the availability and quality of water resources in a given locality or region in order to ensure that supplies are available to accommodate existing and future water consumers. Failure to do so can result in growth limitations, inequitable development, heated public debate, and litigation regarding usage priorities. In order for the energy industry to be environmentally responsible, technologically ready, and economically stable, advanced research to explore and resolve water issues and their relationship to CCS is imperative.
—J.P. Ciferno ([email protected]) is technology manager, existing plants, Emissions & Capture, Office of Coal and Power R&D, National Energy Technology Laboratory, U.S. Department of Energy. R.K. Munson ([email protected]) and J.T. Murphy ([email protected]) are senior engineers with Leonardo Technologies Inc. B.S. LaShier ([email protected]) is a scientist for Booz Allen Hamilton.