Water chemistry an important factor to consider for cycling HRSGs
Combined-cycle plants will continue to be cycled on and off frequently as long as natural gas costs more than coal. The need to start a plant’s gas turbine quickly—to meet short-term electricity demand—sometimes overshadows the potential effects of cycling metal temperatures of heat-recovery steam generator (HRSG) tubes and those of superheater and reheater headers. In particular, the combined effect of this thermal cycling on water and steam chemistry can be significant.
A metallurgical penalty must be paid each time an HRSG goes through a thermal cycle. Many of the physical damage mechanisms have been addressed in POWER (October 2002; March, April, and September 2004; and March 2006) since the boom in combined-cycle plant construction began over a decade ago (Figure 1).

1. Fast mover. Heat-recovery steam generators like this one are now cycled often, sometimes daily, to meet swings in demand. Water chemistry has to keep up with changes in HRSG operating conditions. Courtesy: David Daniels
Excessive HRSG cycling can result in physical damage to the unit and increase the frequency of corrosion and tube failures. But there is another less-appreciated, but nonetheless important, chemistry-related variable in the failure equation. Disruptions in HRSG water chemistry are caused by various actions. Some are expected and others are artifacts of unit cycling. This article discusses both types.
Prevention first
Corrosion products can form quickly if water, air, and metal are allowed to occupy the same space in an HRSG. Proper lay-up is critical to good start-up chemistry, particularly on a cycling unit. Whenever possible, operators should maintain steam pressure on drums and the vacuum on the condenser for as long as possible. Many HRSGs use stack dampers to keep the heat in and bottle up the unit.
HRSGs that are cycled daily typically maintain steam pressure overnight or even over a weekend, depending on the ambient temperature and the condition of the stack dampers. If the unit has an auxiliary boiler, steam seals on the steam turbine can be maintained, to preserve condenser vacuum. This improves the chemistry during start-ups. If an HRSG is to remain off-line long enough that all pressure will be lost, drums and steam piping should be filled with water and blanketed with nitrogen.
Obtaining proper samples
As a combined-cycle plant is cycled, it’s difficult to know exactly what’s happening inside the HRSG. Flow disruptions in sample lines (reduced flow, or swings due to turbulence in the lines) can cause measurement errors. Disruptions also can be caused by on-line water-chemistry analyzers, particularly if they are not protected during shutdown. In many cases, sample panel inspection is a low priority for operators during start-ups.
Another problem is the sample itself. Thermal gradients in water walls can cause metal oxides to become entrained in the sample. In addition, oxides that have collected in the ends of the drums or in the headers can become entrained in the sample during start-up. These solids can produce two effects.
The first is strictly physical: The solids can block or partially block the sample line to analyzers. Although rod-in-tube-style pressure-reducing valves—such as VREL units from Sentry Equipment Corp. (www.sentry-equip.com)—are now common in HRSGs, they often are not exercised when the unit is starting, so solids accumulate in the line. This effect can be exacerbated by reduced flow though the sample lines, which allows solids to settle even during operation. The second effect is chemical: solids can change the chemistry of the sample by either absorbing or releasing salts into the sample, as a function of its temperature and pressure.
One of the most important but overlooked steps in obtaining a representative sample is to adequately blow down the sample lines prior to valving the sample lines directly to the analyzers. The blowdown flushes out contamination in the sample line, so the sample reaches the panel cleaner and more quickly. A good rule of thumb for line cleanliness is that waterwall samples should flow at a minimum of 6 ft/sec. Check your in-line filters to make sure they are not constraining flow to on-line analyzers.
The performance of an on-line analyzer can be adversely affected when no sample stream is available (while the unit is shut down) or when samples are contaminated by metal-oxides during start-up. Analyzers that measure pH, dissolved oxygen (DO), silica, or sodium are particularly vulnerable to either condition. To keep analyzers from running dry, some plants valve deionized water to them and sample panels. Even if an analyzer doesn’t run dry, the sample pressure it receives during start-up is less than normal for the drums. As a result, samples may not flow properly until they come up to pressure. Some panels now include motor-operated VREL pressure-reducing valves that automatically adjust to different pressure conditions, always maintaining the set flow rate.
Consider start-up transients
High levels of DO, particularly during start-ups, increase the potential for corrosion fatigue in HRSGs. The other risk factor for this common HRSG failure mechanism is thermal stress, which is more prevalent on cycled units.
The ability to remove DO from the hotwell and from cold, fully oxygenated makeup water may be hampered by design factors. Among them are the absence of a stand-alone deaerator, the lack of an auxiliary steam source to deaerate water prior to start-up, and the inability of the condenser to remove DO from significant amounts of cold makeup water.
A case study in HRSG cycling chemistry
Some of the effects of cycling operation on water and steam chemistry were seen in a controlled cycling test performed on a 2 x 1 combined-cycle power plant. Chemistry was among a variety of parameters that were monitored, including temperatures, pressures, and flows. In this test the units went through two back-to-back cycles as the units came off-line around midnight and were restarted at 4:30 a.m. The plant was experiencing moderate nighttime temperatures typical of Texas in March when a series of cycling tests were performed. The outages represented the quick start-stop type of cycling that many HRSGs experience.
Chemistry parameters were closely monitored when the units were restarted. Because pressure remained on the HRSG and steam piping, the sample panel was not removed from service during the outage. The plant has an auxiliary boiler that provides steam to the steam seals on the turbine when the HRSGs are off-line and maintains vacuum in the hot-well at all times. This minimizes the formation of corrosion products in the condenser and keeps the water in the hotwell deaerated.
On these units, the amount of DO in samples taken at the condensate pump discharge (CPD) swung between 200 ppb and 400 ppb for about 4 hours after the units were started up. (When baseloaded, these units have dissolved oxygen readings consistently below 10 ppb.) During this period, high (>100 ppb) levels of DO were even noted at the suction of the boiler feed pump, suggesting that DO was present throughout the low-pressure (LP) sections of the HRSG.
During the tests, engineers noted that significant fluctuations in DO at the CPD occurred every time water from the condensate storage tank was added to the hotwell (Figure 2). Major spikes also occurred while the units were off-line and as makeup water was added to the hotwell, while the condensate pumps remained running. These high DO values persisted after the units were restarted, and smaller spikes occurred every time makeup water was added to the hotwell. Typically, similar plants have no special piping or provisions for deaerating the oxygen-saturated makeup water. At this plant, the condenser does have a steam sparging system, but it was designed to be used for freeze protection, not for oxygen removal.
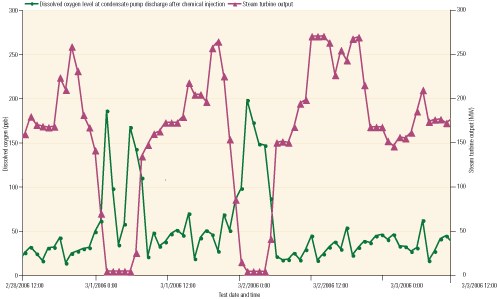
2. That sucking sound. This graph charts dissolved oxygen content at the boiler feed pump outlet during start-up of an HRSG. Source: David Daniels
If enough DO isn’t removed from the condensate of a cycling unit, more must be removed from makeup water before it enters the hotwell. Because HRSG materials are all-ferrous, it is neither necessary nor desirable to add an oxygen-reducing chemical (an oxygen scavenger) or to create a chemically reducing condition. Oxygen scavengers increase the rate of flow-accelerated corrosion (FAC) in feedwater, economizers, and LP evaporator piping. That’s not a good idea, because some HRSG designs are inherently prone to FAC. Adding chemicals such as hydrazine and carbohydrazide only makes the situation worse.
On the other hand, the combination of excessive DO, particularly during start-up, and low-pH feedwater fosters corrosion fatigue in HRSG tubing. Accordingly, it is desirable to prevent, if at all possible, oxygenation of the water in the hotwell and to control the level of DO in the makeup water used during start-up.
Removing DO from makeup, particularly on HRSGs that have been laid-up dry, can be a challenge. Passing makeup water through gas transfer membranes (POWER, September 2005), sparging condensate storage tanks with nitrogen, and catalyzed removal of dissolved oxygen are all proven methods for removing oxygen from cold, high-purity water. In some cases, a combination of technologies may be required.
When the condenser is under vacuum and hot, some removal of DO from makeup water occurs naturally. But the effect is limited. For example, at the combined-cycle plant we’re studying, opening the makeup valve quickly swamped the hotwell with DO, which the boiler feed pumps then carried to the HRSG. To solve the problem, plant engineers adjusted the opening rate of the makeup valve to the condenser. By controlling the rate at which oxygenated water is added to the hot-well, the level of DO in the hotwell now is maintained within reasonable limits.
Maintaining sample integrity
Steam chemistry also can be affected by cycling. Here again, the challenge for operators is to maintain sample integrity and continuity. As the generating unit cools, the flow of samples of main steam or reheat can be lost. During the subsequent restart, particles of iron may flake off pipe walls, and salts present in superheated areas of piping can solubilize and affect sensitive readings. Cation conductivity readings, for example, don’t just indicate levels of chloride and sulfate ions. They also are sensitive to carbon dioxide produced by any organic contamination. Readings from sodium monitors are equally sensitive to changes in the flow and pressure of the steam sample line.
Figure 3 shows measured values of cation conductivity and sodium levels of main steam samples taken during cycling operation of the unit. Determining how much of the spike at right was due to a sampling anomaly and how much really represents contamination would require species-specific evaluation methods such as ion chromatography.
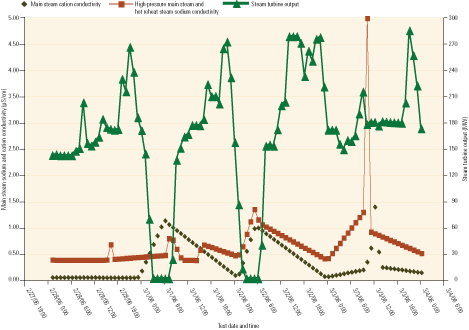
3. Key indicators. This is what main steam chemistry looks like during cycling operation. Source: David Daniels
It’s important to note that the spike in cation conductivity required hours to return to normal. In the back-to-back cycling case study, the cation conductivity had not returned to a baseline level when the unit was restarted. In this situation, there was no contamination from feedwater or any reason to expect contamination from an outside source.
The importance of cation conductivity in the early detection of condenser tube leaks cannot be overstated. Operators and chemists should be concerned when these high values appear during a start-up. Ignoring them only makes the situation worse. Doing so could make you miss the early signs of a major contamination event, such as a condenser tube leak.
Phosphate hideout
Nearly all the HRSGs used for power generation contain all-steel components and no copper alloys. As mentioned, there is typically no reason for operators to use oxygen scavengers for HRSG water treatment. Although all-volatile treatment is recommended for HRSGs, some operators feel more comfortable maintaining a small phosphate residual in their units’ high-pressure (HP) and intermediate-pressure drums.
Cycled HRSGs may see an increase in phosphate levels during start-up due to precipitation of phosphate species on the walls of certain sections, particularly the HP evaporator. This phosphate may or may not be accompanied by a change in the pH of the sample.
Only trisodium phosphate should be used as the source of phosphate for any HRSG. If a significant drop in pH (of one unit or more) accompanies an increase in phosphate concentration, it can be assumed that the phosphate is reacting with magnetite on the HRSG tubing and that phosphate gouging is a possibility. If that is the case, the phosphate concentration during normal operation must be reduced. Experience suggests that it is not a good idea to feed phosphate during duct firing, due to the increased heat flux in the HP evaporator during this operation.
As these units were restarted, the phosphate concentration increased to 5 ppm in the HP drum (normal is between 0.5 ppm and 1.0 ppm). Specific conductivity also increased while the unit was off-line and then returned to normal when the unit was restarted. During the cycling, the pH in the HP drum decreased slowly on both units over a number of hours, even after the units were back up and operating. Here again, the difficulty is differentiating between the actual conditions in the HP drum and the interaction of the sample with magnetite in the sample line. The pH analysis, in particular, can be affected by conditions in the sample lines.
Silica standard
As a cold steam turbine is started up, some silica is rinsed off the turbine blades and back into the condensate in the hotwell. Unless polishers are in place, this will temporarily increase the level of silica in the evaporator and silica will be re-entrained in the steam. The silica level in main steam or hot reheat steam should be less than 10 ppb during normal operation. At this level, silica deposits should not accumulate to the point that they become a significant issue between turbine outages. Higher levels of silica in the steam during startup may be a concern. Here again, the question is, What is real and how important is it if it is real? When dispatch needs the power "now," plant operators have to realize the consequences of the choices they make.
On-line silica analyzers, although generally very accurate and reliable during operation, can be affected by sample flow and particulates that accumulate in the sample filter just upstream of the analyzer. Analyzers and filters also may be affected by long shutdowns. For seasonally operated HRSGs (that will remain off-line longer than three weeks), the reagents should be removed from the analyzer and the analyzer flushed out with deionized water multiple times. To prevent potential problems, some utilities pipe a continuous flow of deionized water to the analyzers when the unit is off.
If silica is monitored directly from the steam sample, it is important that this value and the value in the HP drum correlate to the known chemical volatility of silica in steam at specific pressures (see table). We tend to forget that parts per billion is really a very small concentration, so many factors can affect the reported result. When in doubt, it is usually better to rely on the drum silica sample result, rather than the steam silica value, during start-ups, as the value is much higher and less prone to sampling errors.

Predicting silica concentrations. Allowable silica content in an HRSG’s high-pressure drum for producing saturated steam with 10 ppb silica. The data assume a drum pH of 9.0. Source: David Daniels
—David G. Daniels is a principal of M&M Engineering and a contributing editor to POWER. He can be reached at [email protected].