Techniques for Determining Limestone Composition and Reactivity
Limestone composition and reactivity are critical factors that determine the performance of limestone-based wet flue gas desulfurization systems. Limestone quality affects sulfur dioxide (SO2) removal, reaction tank sizing, limestone consumption rate, and composition of the gypsum product and waste streams. Reactivity is a direct measure of how readily a limestone will provide alkalinity to neutralize the acid resulting from SO2 dissolution in water. In this article we review your limestone analytic measurement options and discuss their relative accuracy and limitations.
Limestone is a commonly occurring sedimentary rock, predominantly composed of calcium and magnesium carbonate (MgCO3) with various amounts of silicates, metal oxides, and other impurities. Mineralogically, limestone may be regarded as calcite, magnesian calcite, dolomite, or an aggregate with varying proportions of each of these minerals.
Pure calcite exists as crystals of calcium carbonate (CaCO3) with a rhombohedral unit cell. A limestone becomes dolomitized as magnesium ions are integrated within the calcite matrix, magnesium substituting for calcium on a one-to-one atomic basis. Limited integration of magnesium produces magnesian calcite. Complete dolomitization occurs when an equimolar ratio of CaCO3 and MgCO3 exists within the lattice.
Substitution of MgCO3 into the crystal matrix is an exothermic reaction. As a result, the dolomite and, to a lesser extent, magnesian calcite, are more thermodynamically stable structures than pure calcite. Therefore, the lattice strength is increased as magnesium is incorporated. Like calcite, the basic crystal structure of dolomite is rhombohedral, with magnesium occupying every other position from calcium on the cation plane.
The convention within industry has been to assume that all of the MgCO3 in limestone is dolomitic, with an equimolar molar ratio of MgCO3 and CaCO3. Actually, the molar ratio of these species can vary considerably from crystal to crystal, and the reactivity of each crystal varies in relation to the fraction of MgCO3 in the crystal. Most limestones selected for wet flue gas desulfurization (WFGD) application are highly calcitic, containing less than 5% MgCO3.
Limestone for Sulfur Removal
Accurate determination of limestone composition and reactivity are crucial in the selection of reagents for WFGD applications, especially for systems producing commercial grade gypsum products. (See Seminole Generating Station story, p. 52.) Although systems can be constructed to utilize a wide range of limestone composition and reactivity, limestone quality must be agreed upon early during system design because this parameter affects unit sizing, product quality, wastewater treatment, limestone consumption rate, and WFGD system performance.
Mineral species of interest are calcite, magnesian calcite, and dolomite. The magnesian calcite or substituted calcites range from 6% to 13% magnesium replacement of calcium in the crystal structure. Also of concern are the silicates and metal oxides, which are inert in the WFGD process but affect gypsum purity; concentrations of these inerts in the gypsum are often limited to specific levels for commercial grade gypsum product.
Though the use of lower-quality limestone may provide an advantage in system operating cost, the benefit of these savings should be weighed against impacts on system performance. Limestone of acceptable quality is necessary for achieving design-level performance.
WFGD System Chemistry
Pulverized limestone is used in WFGD absorbers to provide alkalinity for reaction with SO2 from the flue gas. Within a WFGD system, SO2 dissolves into the aqueous slurry, where dissociation occurs and produces sulfurous acid. Acid undergoes a heterogeneous reaction with the surface of limestone particles, yielding calcium cations and hydrogen carbonate anions within the slurry. The hydrogen carbonate then undergoes acid-base neutralization, producing carbon dioxide (CO2) and water. Within the absorber reaction tank — in addition to limestone dissolution, acid neutralization, and CO2 stripping — sulfite is oxidized to sulfate, and the calcium ions, sulfate, and water subsequently react to form a gypsum product.
Gypsum composition and purity are dependent upon limestone composition. When limestone possessing low available CaCO3 is fed to the system, limestone feed rates must increase to provide the same level of SO2 removal. In so doing, both limestone consumption and the level of impurities, including CaCO3, within the slurry will increase. Consequently, the gypsum purity and quality will decrease. Several means of determining limestone composition are available.
Thermogravimetric Analysis (TGA). TGA is an analytical technique that can be used to determine the proportion of different mineralogical species within a limestone sample. This method harnesses the differences in thermal decomposition temperatures of limestone constituents to determine composition by measuring weight loss as a function of temperature. As a limestone sample is heated in a stream of CO2 gas, its weight decreases as decomposition occurs from the increased temperature. Sample weight loss is interpreted as a weight fraction of CO2 evolved, by taking the ratio of sample weight to the total original sample weight.
Mineralogical structure affects the decomposition temperature of a compound. Carbon dioxide is evolved from CaCO3 and from the MgCO3 bound within dolomite and magnesian calcite at different temperature ranges (Table 1). Initially, water, organics, and impurities are driven off at low temperatures. After this point, decomposition of the carbonate species within limestone begins.
![]()
Table 1. Approximate decomposition temperatures for limestone. Source: B&W
It’s important to note that magnesium may be bound within the dolomite or as magnesian calcite. However, precise quantification of the magnesium content within the magnesian calcite phase by TGA is difficult due to the spectrum of magnesium concentrations that may exist within magnesium-substituted calcites and due to concentrations within the sample approaching the detection limit of the TGA. A strong decrease in weight is often associated with CO2 loss from the MgCO3 within the dolomitic structure. After CO2 loss attributed to MgCO3 species, CO2 is evolved as from decomposition of CaCO3, irrespective of mineral phase.
Analysis of TGA data is based upon sample weight loss as a function of temperature and the derivative of this function (Figures 1 and 2). The total sample weight loss is calculated on a CO2 basis, providing a direct measurement of total carbonate within the sample.

1. TGA data for NIST SRM 1d. Source: B&W
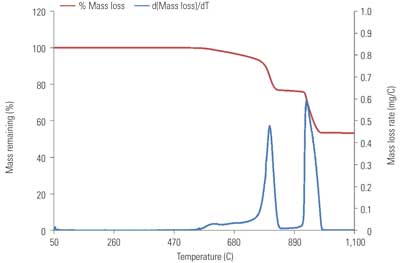
2. TGA data for NIST SRM 88b. Source: B&W
The derivative of the weight loss vs. temperature function provides a means to quantify the CO2 associated with magnesium species. The dolomitic peak is integrated to afford a value for the CO2 weight loss assigned to dolomitic MgCO3. Due to its low concentrations in limestone, the MgCO3 associated with magnesian calcite seems to be outside of the TGA detection limit and indistinguishable from the baseline of this derivative function for most limestones. The difference between the total CO2 weight loss and dolomitic MgCO3 weight loss is attributed to CaCO3 decomposition. Such an approximation entails grouping the MgCO3 associated with magnesian calcite into the value for total CaCO3.
X-Ray Fluorescence (XRF). XRF is a widely available, rapid, and cost-effective technique for elemental analysis. It also offers the benefit of quantifying many elements simultaneously, including such species as silicon and various metals.
During XRF, a specimen is irradiated by a high-energy X-ray beam. Energy is released in wavelengths characteristic of specific elements. The total energy released in each characteristic wavelength is proportional to the amount of that element present. These wavelengths are used to identify the elements present in a specimen. Concentrations of the elements are determined by relating the measured radiation to analytical curves from reference materials of known composition.
Wet Chemical Test Methods. Wet chemical test methods are used in combination to provide results that could be obtained from a single XRF or TGA analysis. These methods are often labor-intensive and more expensive than the other techniques discussed.
The TAPPI train is a gravimetric methodology for determining the total CO2 fraction of a limestone sample. Oxalate titration is a method for determining the calcium content of a limestone sample. Ammonium phosphate gravimetric methodology is used to determine the magnesium content of a limestone sample.
Laboratory Testing Results
To compare the accuracy of different techniques for limestone analysis, a dolomitic limestone control (NIST SRM 88b) and an argillaceous limestone control (NIST SRM 1d) were selected for testing by TGA, XRF, and wet chemical test methods. NIST SRM 88b has a relatively high MgCO3 content and exhibits low reactivity due to the dolomitic content. In contrast, NIST SRM 1d is highly calcitic and would be more representative of a WFGD reagent limestone. Multiple analytical runs were conducted on each of these limestone samples using the various techniques.
Blended samples of NIST SRM 1d spiked with NIST SRM 88b were created for TGA and XRF analysis, such that low quantities of the dolomitic stone were mixed into the argillaceous limestone, creating artificial samples with a range of dolomitic magnesium carbonate content. MgCO3 content of these blends ranged from 1% to 4% by weight. These low percentages of MgCO3 are representative of those found in limestones used in field units.
Use caution when comparing results from different techniques because the techniques use different physical properties of the limestone and associated assumptions, yielding different bases for quantification of individual species, some of which may be perceived as a natural bias when reviewing results.
Elemental techniques, including XRF and wet chemical methods, provide values for the total fraction of an elemental species. However, information about mineral structure is not obtained. When interpreting XRF or wet chemical results, assumptions are made by the WFGD system supplier as to magnesium carbonate partitioning within the limestone.
TGA is a destructive technique, in which differences in thermal decomposition temperature of CaCO3, dolomitic MgCO3 and MgCO3 not bound within the dolomitic matrix are used during data interpretation to discern both mineral and chemical composition. Although it quantifies the fraction of crystal structures in the bulk sample, TGA cannot yield information as to the exact elemental composition.
Determine Total Calcium Carbonate. Quantification of total CaCO3 is necessary because this is the main limestone constituent that will react in a WFGD system. Inaccuracies in this measurement translate into errors in calculating total available CaCO3, which will affect the system design and performance. To compare the accuracy of each technique, testing was performed on two NIST-certified standard limestones and on six blends of these limestones (Table 2).
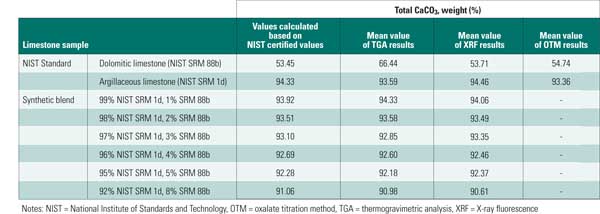
Table 2. Testing measurement accuracy. Mean CaCO3 testing was conducted to compare the accuracy of each measurement technique on two NIST-certified standard limestone samples and on six blends of those limestones. Source: B&W
The results showed that all methods provided an accurate estimate for the total CaCO3 content of NIST SRM 1d. For 88b, XRF afforded the most accurate measurement of total CaCO3, as compared to certified values. Results using the oxalate titration method were slightly higher than expected, but they were allowable. TGA results were biased high for this sample. Some of this overestimate was likely caused by the assignment of magnesium in the magnesian calcite phase to total CaO. Highly dolomitic stones are unlikely to be used in WFGD systems. Therefore, though TGA may have a bias in this range, it is an appropriate method for limestones with magnesium content in the range encountered in commercial WFGD application.
Determine Magnesium Carbonate Content. Determining magnesium content is another important aspect of limestone compositional analysis. This testing approach illustrates the fundamental differences between TGA and elemental techniques, including XRF and wet chemistry. XRF and other elemental techniques provide a value for magnesium based upon the total magnesium content detected within the species, expressed as MgO or MgCO3. Industry convention is to assume that all MgCO3 reported from elemental analysis is bound as dolomite in a 1:1 atomic ratio with CaCO3.
In contrast, TGA is a physical technique that yields different results depending upon the degree of dolomitization of the limestone sample, as CO2 will be evolved from highly calcitic and highly dolomitic mineralogical phases at different temperatures. Therefore, results for the dolomitic magnesium carbonate content reported by TGA should be lower than XRF results for total magnesium, expressed as MgCO3. This lower value results from the difference between the total MgCO3 and the MgCO3 strongly bound in a dolomitic matrix, likely equating to the magnesium bound as magnesian calcite.
All three methods underestimated the total MgCO3 content of the NIST 1d. This may be due to the very low concentration of MgCO3 present in this sample. The six control blends were created to address this concern, exhibiting a range of MgCO3 content. XRF and the ammonium phosphate gravimetric method afforded similar results for the dolomitic limestone, agreeing well with certified values. TGA results were lower for the dolomitic limestone, as this method quantifies dolomitic MgCO3 rather than total MgCO3 content.
We observed that average XRF results provide a better indication of total MgCO3 when compared to TGA results, though some of this may be attributed to differences in mineral phase (Table 3).
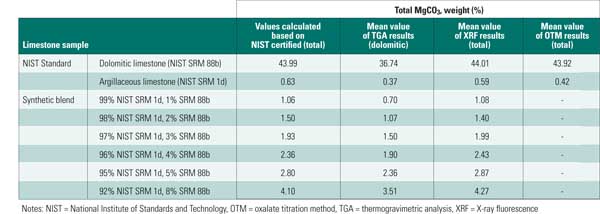
Table 3. Mean MgCO3 test results. Source: B&W
Overall, XRF provided accurate estimation of the total magnesium carbonate content for these samples, but it could not provide an indication of mineralogical phase. TGA results provided an acceptable estimation of dolomitic MgCO3 content. Additional development of TGA would be required for quantification of the magnesium in magnesian calcite.
Determine Total Available Calcium Carbonate. The primary purpose of obtaining compositional data about the CaCO3 and MgCO3 content of a given limestone is to determine the available CaCO3. Available CaCO3 is the amount of CaCO3 within a limestone expected to react within a WFGD scrubber.
Total available calcium carbonate is calculated by subtracting the dolomitic carbonate contribution from the total carbonate of a sample. Total available CaCO3 is calculated from TGA results by subtracting one molecule of CaCO3 for every molecule of dolomitic MgCO3 measured. Because magnesian calcite is credited toward total CaCO3, this alkalinity would be credited toward total available alkalinity. In contrast, indication of mineral phase partitioning is beyond the capability of elemental analysis. Therefore, the convention is to assume that all the magnesium exists entirely as dolomite, ((Ca,Mg)(CO3)2), with a 1:1 ratio of CaCO3 and MgCO3. This fundamental difference is important to recognize but may not be significant due to the low concentrations of nondolomitic magnesium carbonate in many WFGD reagent limestones.
Analyses of filtrates from WFGD slurry samples indicate that dissolved magnesium concentrations range from several hundred to several thousand parts per million. The concentration is very dependent on the chloride content of the coal and the chloride concentration maintained by the purge stream. However, it is apparent from this data that considerable MgCO3 is dissolved in WFGD systems. In some cases, utilities will provide a value for MgCO3 utilization; typical values range from 20% to 35%. Research and development projects aimed at developing a correlation for predicting the available MgCO3 are in progress.
TGA and XRF provide excellent agreement with expected values for total available CaCO3 (Table 4). TGA results were slightly higher for most samples than XRF or the NIST-certified values. Much of this deviation may be attributed to the magnesium credited toward available alkalinity by the TGA method.
TGA did provide an overestimate of the total available CaCO3 in NIST SRM 88b. This deviation could be due to assignment of some of the total MgCO3 toward the total alkalinity, or the bias in total CO2 evolved observed for this sample. Both TGA and XRF are acceptable for determination of the total available CaCO3 in limestones used for WFGD.
Limestone Reactivity Testing
Determination of limestone reactivity is essential for accurate modeling and design of WFGD systems. Because reactivity is a measure of the rate at which a limestone will provide alkalinity to react with the acid created during SO2 dissolution and hydrolysis, it is used in estimating the amount of limestone that must be fed into the absorber to maintain a given pH for a particular Ca/S stoichiometry and solids residence time. This data can then be used in the initial design of the WFGD system.
However, when a limestone possessing lower than design reactivity is used, feed rates would increase over those developed for the reactive limestone in order to maintain pH and SO2 removal. As a result, gypsum purity would decrease due to increased inerts and unreacted CaCO3 and MgCO3 fed to the dewatering system. The flow rates of waste streams may also increase.
Currently, an industry standard for reactivity is not available, and a number of different procedures exist for conducting reactivity measurements. A test method is under development by ASTM; Babcock & Wilcox (B&W), utilities, limestone suppliers, and other original equipment manufacturers are supporting this effort by serving on the task group. The following test method was developed by and are currently used by B&W to conduct limestone reactivity analysis:
-
Sample preparation. Repeatable sample preparation is essential, as differences in limestone grind will produce different initial particle size distributions (PSD).
-
PH and automatic titrator control settings. Tight system control should be employed to ensure constant pH and controlled acid addition. Undershoot and overshoot of pH are to be minimized because swings in pH will change the reaction rate, rendering results inconclusive.
-
Acid. Because direct reaction between limestone and the anion species does not occur once the acid has dissociated, hydrochloric acid (HCl) may be used for limestone titration. The resulting products remain soluble, leaving unreacted limestone and inert species as the only particulates within the solution. When sulfuric acid (H2 SO4) is used as the titrant, gypsum product is produced, precluding measurement of limestone PSD after the start of reaction and leading to the potential for electrode scaling and fouling.
-
Common ion concentration. Due to the principles of chemical equilibrium, the rate of the reaction is affected by the relative concentrations of products and reactants within solution.
-
Wetting agent. Use of a wetting agent ensures that all of the sample surface area is available for heterogeneous reaction. Ideally, the wetting agent also acts as a dispersant, reducing error potential from sample agglomeration.
-
Correction for initial PSD. B&W has tested the same limestone that has been ground to different degrees of fineness. Results indicate that the empirically determined value for the reaction rate will not be constant unless it is corrected for PSD.
Reactivity Testing Methodology
The B&W limestone reactivity test method is used to ascertain the rate constant of a limestone sample and to qualify candidate limestones for suitability as WFGD reagents with regard to this parameter. This method uses an automatic titrator to add HCl to a fixed amount of limestone sample. The automatic titrator includes an agitator and provides precise control over the titration, which is necessary when determining the rate of reaction. PSD measurements are taken to provide an estimate of the limestone surface area available for reaction. Titration results and PSD measurements are used to estimate the reactivity constant of a given limestone. Results are compared to a limestone of known field performance.
To prepare the sample, the limestone is pulverized in a disk mill and screened such that 100% passes through 325 mesh. By using a constant mill setting during grinding, and by screening the sample, some control over PSD is afforded. However, obtaining two samples with the same PSD is highly unlikely, even from the same screening. This is primarily due to differences in the Bond Work Index of the different limestone samples. Double screening is not used due to concern that error may be induced through such activity as, theoretically, fractions of differing composition may result above and below the smallest screen.
Within a WFGD system, limestone is thought to react with the hydrogen ions in solution resulting from SO2 dissolution and its subsequent hydrolysis in water. Because limestone does not react directly with SO2 but, rather, reacts with the protons released from its absorption, any acid may be used to model the reaction of limestone and acid. B&W chooses to use HCl for limestone reactivity testing because the products of this reaction remain soluble in water, affording PSD measurements at later stages in the reaction.
A 1-gram sample of the prepared limestone is titrated with HCl in an automatic titrator until complete reaction at a pH of 3.85. Use of a compatible wetting agent is critical to allow wetting of the limestone sample and to ensure complete dispersion of the limestone sample particles. The volume of acid added is recorded, and this result is used to determine total alkalinity of the limestone sample. From analysis of the titration results, the amount of HCl required to titrate 60% of the total alkalinity is calculated.
A second 1-gram sample from the same screening is prepared and titrated at a pH of 5.0 until the calculated volume of acid required to react 60% of the total alkalinity has been added. Results are recorded as volume of acid added as a function of time (Figure 3).
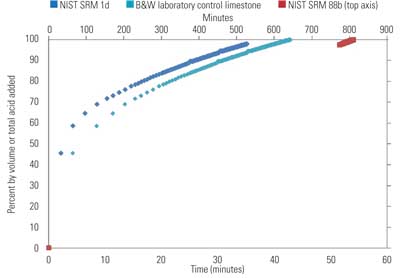
3. Volume of acid added vs. time for 60% titration of a limestone’s alkalinity. The NIST SRM 88b sample data uses the secondary axis time scale located on top. Source: B&W
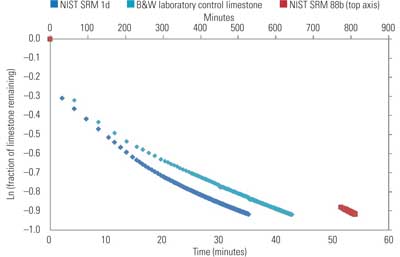
4. Natural log of the fraction of limestone remaining vs. time. The NIST SRM 88b sample data uses the secondary axis time scale located on top. Source: B&W
During data analysis, the 60% titration data, original sample mass, and total alkalinity of the sample are used to construct a curve for the natural log of the limestone fraction remaining as a function of time (Figure 4). The derivative of this curve at the endpoint of titration is used in calculation of the reaction rate constant. PSD measurements for the sample are obtained at the titration endpoint to correspond with this slope.
A PSD reading is then taken on an aliquot of titrated sample. The initial and inert PSDs are also measured. The PSD readings are used to determine the specific surface area, expressed as the Sauter mean diameter (SMD), of the alkaline species in the sample at the titration endpoint. The alkaline particles are assumed to be spherical. The SMD and the rate of acid addition are used to determine a reaction rate constant with units of microns of alkaline particles reacted per minute. The rate constant is independent of the actual particle size.
The B&W laboratory control limestone is a WFGD reagent limestone of known field performance, and this stone reacted in a similar period of time to NIST SRM 1d. In stark contrast, the dolomitic control limestone required almost 14 hours to reach 60% reaction at a pH of 5.0. Such a limestone is not suitable for use in WFGD systems.
Reactivity results for these limestones, and for reagent grade CaCO3 normalized to the B&W control limestone, are provided in Table 5. Large variances in reactivity between the control stone, argillaceous limestone, and reagent grade CaCO3 are thought to be attributed to differences in initial particle size distribution and sample preparation methods, as all of these should provide adequate performance in a WFGD system. Observe that the dolomitic limestone only exhibited 1% of the reactivity of the B&W control limestone, indicating that it would be unreactive in most WFGD systems.

Table 5. Relative reactivity of various limestones. Source: B&W
Testing to improve this method continues, with a primary focus on developing a correlation for initial particle size distribution. Preliminary results for the relative reactivity of samples of the same limestone ground to three different particle size distributions indicate that initial PSD may have significant impact on the reaction rate constant, as empirically determined from typical acid titration based reactivity methods.
XRF results provided by Don Broton, senior scientist of CTL Group Inc.
Normal
0
0
1
29
170
1
1
208
11.0
0
0
0