Focus on Organics in Steam
Organic compounds can enter the steam cycle from a number of sources, including water treatment chemicals, or as part of a manufacturing process. Regardless of the source of the organics, their effects range from fouling polisher resins to causing significant steam turbine damage. Conventional water pretreatment systems are available to remove organics from water, but removing organic compounds at their source is the best place to start addressing the problem.
The presence of organic chemicals in feedwater and steam has been a power industry issue for a number of years and has been the subject of previous POWER articles (see "Organics in the Boiler and Steam: Good or Bad?" September 2006), as have the effects of organic compounds on the cation conductivity of feedwater and steam ("Cation Conductivity Monitoring: A Reality Check" May 2008).
Given the pervasiveness of these concerns, it pays to be aware of the latest understanding of how organics affect a power plant’s steam cycle and how their effects can be mitigated. That was the focus of the third conference on the interaction of organic compounds with water and steam, held in November last year in Lucerne, Switzerland. The conference was attended by90 participants from 19 countries and included 30 presentations over two and a half days on various aspects of the effect of organic compounds on water and steam to generate power. This article presents some conference highlights and current thinking about managing organics in the steam cycle.
Organics 101
Three major sources of organic chemicals in utility steam generating systems are:
-
Natural organic matter (NOM), which consists of organic chemicals that pass through the pretreatment equipment and wind up in the boiler feedwater and steam.
-
Treatment chemicals added to the feedwater that volatilize into the steam.
-
Contaminants contributed by equipment such as condensate polishers.
Organic compounds, such as lubricating oils, can also contaminate the boiler, but these instances are rare. Each of the major sources poses a different risk for plant equipment.
The most common problem with organic compounds in the steam cycle is their detrimental effect on cation conductivity. In essence, heat and temperature in the steam cycle break down large and sometimes non-ionic organic compounds into shorter carbon molecules, and often carboxylic acids, which are ionic. Organic chemicals that create anionic species (acetate, formate, and dissolved carbon dioxide) in the steam or condensate will contribute to cation conductivity. Their presence makes it difficult for the operator to know if contaminants such as chloride or sulfate are in the steam, and therefore pose a risk to the turbine. It’s like driving in a rainstorm with bad windshield wipers — poor visibility prevents you from identifying a hazard early enough to respond.
Improvements in demineralized water purity, and the ability to improve the detection of contamination, have allowed turbine original equipment manufacturers (OEMs) to define a new "normal" for a steam cycle operating with no contamination from chloride and sulfate, thus lowering the acceptable cation conductivity. These lower limits leave little if any room for the contributions from carbon dioxide or other organic breakdown products.
For this reason, owners may void their warranty with the turbine manufacturer if their plant operates above this limit unless they can prove that the cation conductivity is not coming from chloride or sulfate.
Limiting organics can be a particular problem during commissioning, when there may be traces of contamination in the piping that can take a long time to remove.
Complicating matters further, industry studies have examined steam turbines that operate with cation conductivity that is consistently higher than EPRI and turbine manufacturers’ recommendations. The results showed that these turbines did not have a higher rate of turbine-related failures than their counterparts that operate at levels of cation conductivity that are within the current guidelines.
What Is Natural Organic Matter?
Natural organic matter consists primarily of large, complex organic molecules that are the by-product of a living organism. NOM can be humic acids from decaying plant material or polysaccharides created by bacteria in the water.
If the water source is treated effluent from a sewage treatment plant or a source of potable water, it’s likely that the water is chlorinated before it reaches the plant. Chlorinated organic compounds are a particular concern, because they can pass through water treatment equipment only to break down in the boiler, where the amount of chloride in the steam will increase the potential for corrosion on the turbine. These halo-organic compounds present a clear risk to plant equipment.
Documented damage to steam cycle equipment directly attributed to organic compounds is associated with various sources of NOM, some of which have included halogenated organics. Damage has included stress corrosion cracking of turbine rotors, corrosion of carbon steel anchor strips at the base of the turbine blade in the final rows of a low-pressure (LP) turbine, tube failures in a steam generator (chlorinated organic compounds), and corrosion in the LP evaporator tubing in a heat-recovery steam generator.
Many OEM makeup water guidelines give hard limits, such as 300 ppb total organic carbon (TOC). This kind of hard limit is not helpful in solving many of the water treatment challenges facing the power industry. First, the TOC value does not differentiate between different sources of organic compounds. It is important to know the particular source and type of organic compound, as some pretreatment equipment is more effective at removing specific types of organic compounds and less effective at removing others. For example, one utility in the Netherlands saw a jump in cation conductivity when its makeup water source changed, introducing more polysaccharides into the water that were not effectively removed by its pretreatment equipment.
For some units, 300 ppb may be far too high, particularly where there is potential for the TOC to contain chlorinated or sulfonated compounds. For another unit, the pretreatment equipment required to meet these low limits may be extensive and of questionable benefit.
Many water treatment experts are of the opinion that no single limit on organics in makeup water should be applied to all units because many factors determine how much of the organic loading of a steam cycle presents an unacceptable risk. Critical questions to ask before determining an limit include these: What is the source of the organic compounds? What are the operating conditions (temperature and pressure) of the unit? Is it baseloaded or cycling? Will the steam be condensed in the steam cycle, or will most of it go to the steam host? What is the potential for other ions, such as chloride or sulfate, to be associated with the organic compounds? These and similar questions should be answered prior to deciding how and to what extent makeup water needs to be treated for organic removal.
Not only can NOM directly affect a plant, but it also can create problems in equipment, which in turn creates many operations problems. Organic molecules have a long history of fouling anion resins, resulting in shorter run lengths and silica leakage. Cation resins can also be affected by NOM. Organic compounds absorbed onto cation resin have been shown to cause degradation of the resin that results in the release of a different organic contaminant, namely polystyrenesulfonates. So, although the NOM may not contain sulfonated compounds, it may cause increased levels of sulfate in the steam.
Conventional wisdom assumed that NOM would quickly break down into carboxylic acids such as acetic acid and carbon dioxide. Recent testing performed with humic acid at 2,600 psig and 1,022F found that NOM also formed significant percentages of larger and more complex organic molecules in the boiler, even after 48 hours of exposures at these temperatures and pressures. It’s likely that the presence of oxygen in the steam cycle as well as the configuration and recirculation rate on the boiler have a significant effect on the compounds formed and their longevity. Results are likely to be site-specific and may even depend on current operating conditions.
Removing NOM with Pretreatment
NOM has long caused problems with conventional ion exchange demineralizer trains. In addition to shortening run lengths and creating silica in the effluent, organic fouling on the anion beads has been shown to soak up sodium ion during the anion regeneration and bleed it back during operation, increasing the sodium, pH, and conductivity in anion bed effluent. A number of conventional treatments have been examined for their effect on NOM removal (see table).
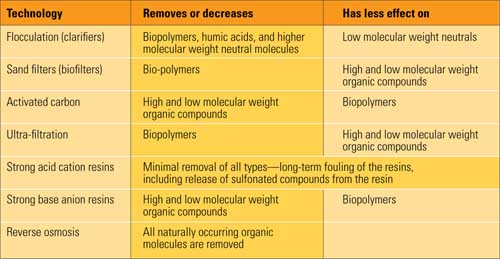
Pretreatment technologies for removing natural organic matter. Source:M&M Engineering Associates Inc.
Ultrafiltration (UF) followed by reverse osmosis (RO) has been proven the best treatment process for removing natural organic compounds in raw water. Although UF does not eliminate a significant percentage of the small molecular weight organic materials, it does remove a significant portion of the particulate organic particles, including the polysaccharides, microbiologically generated molecules. UF also significantly reduces fouling on the RO membranes. Whatever organic compounds are not removed by UF are taken out by the RO. Testing has shown that nearly 100% of all types of organic molecules are removed by RO.
In cases where there is an existing ion exchange train, organic removal is improved by conventional, and some unconventional, treatments. Conventional lime softening removes 20% to 40% of the dissolved organic compounds in the water. Activated carbon filters also remove organic compounds, but not for the reason that most believe.
Organic compounds are initially absorbed on a fresh carbon bed, but the carbon quickly becomes a biofilter supporting bacteria that use the incoming organic compounds as food. Acting as a "biofilter," activated carbon beds can remove a significant portion of some organic compounds. Likewise, multimedia filters can support bacterial growth. Biofilters, however, are not necessarily stable. A sudden influx of chlorine, an extended stagnant period, or a change in the water supply chemistry may result in a sudden release of organic compounds into the water.
The fact that anion resins are so good at absorbing organic compounds has worked to the plant’s advantage when specialized anion resins are placed ahead of their conventional demineralizer specifically for organic removal. The demineralizer bed then must be regularly regenerated with an alkaline brine solution to remove the organics. The combination of an activated carbon biofilter and specialized organic scavenger bed with anion resins can be a simple and cost-effective organic removal system.
Traditionally, RO membranes have preceded ion exchange capacity, but in some cases RO can be used after a conventional cation and anion vessel and before the mixed bed, specifically to remove any organic compounds.
Care should also be taken that the organic chemicals added to water treatment don’t solve one problem and create another. The wrong type, or high feed rates, of coagulants in the clarifier may improve clarity but may leave the clarifier and contaminate the resin beads of the demineralizer.
Organic Feedwater Treatment Chemicals
Currently, the only approved chemicals for boiler and feedwater treatment in the EPRI Cycle Chemistry Guidelines for Fossil Plants are:
-
Ammonia for feedwater pH control.
-
Hydrazine (N2 H4) for control of the oxidation-reduction potential in mixed metallurgy units. (All ferrous units typically do not use hydrazine.)
-
Sodium hydroxide and trisodium phosphate in the boiler water of drum boilers for units using phosphate or caustic treatments.
None of the EPRI guidelines describes suggested chemicals used for water treatment that contain carbon. EPRI and all the major turbine manufacturers rely on cation conductivity as the most reliable indication of the presence of part per billion levels of chlorides and sulfates in steam. In addition, for the large 2,600-psig conventional fossil-fired power plants with consistent high-purity demineralized water and all-ferrous metallurgy, non-carbon-based chemicals have proven to be very successful at maintaining a passive oxide layer in the boiler and preventing corrosion on the turbine.
However, more and more generating stations do not fit this mold. Combined-cycle power plants with air-cooled condensers turn to organic amines to provide a higher pH condensate to prevent corrosion of the air-cooled condenser tubing. There are still many units that have copper-alloy feedwater heaters that will not be replacing their heaters in the near future. These units have long benefitted from the use of amines to protect the copper metallurgy. Amines may also be needed in plants that see some level of NOM in their makeup water to provide an alkaline pH boost to the first condensate in the turbine.
Remember that over the more than 60 years of using neutralizing amines in all types of industrial and utility boilers, to date, no turbine failures can be tied directly to the use of these organic feedwater chemicals. The record includes an extensive history of amine use in pressurized water reactor nuclear steam generators, where amines successfully reduce the rate of two-phase flow-accelerated corrosion.
A variety of neutralizing amines also are used to raise the pH of feedwater and steam condensate. The two most important characteristics of these amines are their basicity and the volatility of the amine. The basicity of the amine determines how much will be required to raise the pH of the feedwater to the desired level. This in turn determines the effect of the amine on cation conductivity. A high basicity amine will be able to produce the desired feedwater pH with less chemical and less cation conductivity. The volatility of the amine determines at what point in the steam cycle the amine will begin to condense.
If protection of the first condensate in the turbine were the desired outcome, then a low-volatility amine would be preferred. If a plant has an extensive network of steam heating coils or sends steam across the fence and recovers and reuses the process condensate, a high-volatility amine is preferred so that the amine will stay in the vapor until it condenses at the end of the pipe. Often, more than one amine is required to cover a plant’s needs. Blends of two and three different amines are common.
Polishers as a Source of Organics in Feedwater
Condensate polishers are the plant’s first and best defense against small amounts of contamination from a weeping condenser tube or demineralizer upset. However, we now know they can also be the source of organic contamination. Full-flow condensate polishers are the rule in nuclear power plants, fossil-fired supercritical plants, and many high-pressure drum units.
Leachable organic compound from new cation resins include monomers of styrene and residuals of organic solvents. As resins age, they leach functional groups from the resin. For cation resins, this includes sulfonated monomers. These can break down in the boiler or steam cycle to produce sulfate. Anion resins tend to leach aliphatic amines, which may be observed only as increased cation conductivity.
New resins leach out higher concentrations of organic compounds than well-used resins. The organics left over from the manufacturing process have a far greater potential for damage. They not only have the capacity to contaminate the process, but they will also foul other resins that contact them.
For example, compounds that leach out of new cation resins will stick to and foul anion resins. Therefore, new cation resins should be regenerated and rinsed separately before they are mixed with anion resins. One expert suggests that new cation resin be regenerated, exhausted with brine, and then double regenerated before it comes into contact with the new anion resin.
Another method is to soak new separated cation and anion resins in warm (120F) demineralized water for four hours or more to remove leachable organic compounds. Each time the strong acid and strong base resins are regenerated, the bead shrinks in the presence of the regenerant. As the resin is rinsed and put into service, the bead swells again. Each shrinking and swelling cycle acts to squeeze some additional level of leachable organic material out of the resin. Extended rinse times may be required not only to reach the desired conductivity but also to remove the leachable organic compounds.
One area where amines may have a detrimental effect is on the polisher resins. Polishers remove acetic acid and other organic breakdown products, but they may also contribute to contamination problems.
Ethanol amine has been a problem with some polisher resins, causing contamination, particularly if the condensate temperatures are high. Carboxylic acids that are absorbed by the cation resin are exposed to sulfuric acid during regeneration, become sulfonated, and be released when the resin is in service. Similarly, organic acids on anion polisher resins can absorb sodium during regeneration and release it during operation. Every organic compound, whether it is an amine or a declumping agent, should be assessed prior to use to see if it will have a detrimental effect on the polisher resins.
To Manage Organics, Know Your Water and Treatment Chemicals
In the future, as power plants contend with poorer quality raw water as source water for their demineralizers, attention to NOM will gain importance. This source of organics has the potential to do damage in the steam cycle, particularly where the organic compounds include halogens such as chlorine. Fortunately, there are treatment technologies capable of removing these organic compounds. Each plant needs to know not only the level of TOC in the raw water but also where those organic compounds originate. This is particularly important when raw water sources are changed. Often, simple steps can be taken to reduce the organic loading on a unit.
Any water treatment chemicals normally added to the steam cycle present a level of risk to the equipment if they are misapplied or overused. With the substantial history of good performance, it would be a shame to summarily dismiss neutralizing amines just because they contain carbon. Properly evaluated and applied, amines and other carbon-based treatment chemicals have the potential to benefit the steam turbine and other plant equipment.
—David G. Daniels ([email protected]) is a principal of M&M Engineering Associates and a contributing editor to POWER.