Combined Mercury and SO3 Removal Using SBS Injection
Though no single mercury capture approach is best for all plants, when you can capture two (or more) pollutants with one sorbent, it’s worth a careful look.
The U.S. Environmental Protection Agency’s (EPA’s) Utility Mercury and Air Toxics Standards (MATS) regulation requires power plants to reduce emissions of hazardous air pollutants (HAPs), including mercury. The regulation requires that these emission reductions be achieved by April 2015, or April 2016 if the plant is granted an extension. Activated carbon injection (ACI) is the most widely used technology for the specific control of mercury emissions; however, ACI’s effectiveness is greatly reduced in the presence of sulfur trioxide (SO3).
This article describes a novel approach for mercury control that relies on the injection of a single sorbent to effectively remove SO3 upstream of the air preheater (APH), which greatly enhances mercury adsorption onto the native “unburned carbon” in the flue gas downstream of the APH. Furthermore, removal of SO3 prior to the APH allows for the flue gas temperature exiting the APH to be reduced, which further enhances mercury capture and improves the plant energy efficiency. The co-benefit capture of hydrochloric acid (HCl) and selenium from the flue gas using this approach is another advantage.
The Challenges of Removing SO3
Over the past decade many generators have installed new emissions controls on their coal-fired power plants, including selective catalytic reduction (SCR) systems for nitrogen oxides (NOx) control and flue gas desulfurization (FGD) systems for SO2 control. One consequence of SCR retrofits has been a significant increase in the amount of SO3 generated in the flue gas and the potential to create a visible sulfuric acid “blue plume.” In addition, higher SO3 levels can adversely affect many aspects of plant operation and performance, including severe corrosion of back-end equipment, fouling of the APH, and limitations in the ability to operate at reduced loads due to SCR operating temperature constraints.
The addition of SCR or FGD to a power plant, or a switch to higher-sulfur fuels, can also trigger the EPA’s New Source Review (NSR) rules. In some cases, power plants have been required to mitigate the elevated SO3 emissions as a result of NSR rules.
The trademarked SBS Injection technology has been widely applied to control SO3 emissions from coal-fired power plants. However, it is only in recent years that the co-removal of mercury has also been documented and demonstrated at several plants.
The technology injects a “sodium-based solution” into the flue gas, typically ahead of the APH or SCR. By removing SO3 prior to these devices, many of the adverse effects of SO3 can be successfully mitigated, and plant performance and reliability can be improved. SO3 removal efficiencies of greater than 98% have been achieved using SBS Injection, with stack emissions typically less than 1.0 part per million (ppm). The process has been installed on 24 boilers representing more than 15,000 MW of generating capacity, and has been in continuous operation for more than 10 years. A typical installation is shown in Figure 1.
As with any emissions control technology, proper design and operation are critical to ensure that desired performance and reliability are achieved. Because the SBS Injection technology relies on the injection of a wet sorbent solution into the flue gas, careful attention during system design is needed to ensure proper atomization and drying of the liquid to avoid solids deposition within the ductwork.
In addition, the injection of any sodium-based sorbent can result in secondary reactions producing sodium bisulfate, which can lead to fouling of the APH. As a result, the injection location must be properly selected to provide adequate reaction time prior to the APH to ensure the SO3 concentration is sufficiently reduced to avoid these reactions. Recent experience has shown that with proper design and operation of the SBS process, these operational issues can be easily overcome.
Mercury Control Challenges
As noted, MATS requires power plants to reduce emissions of HAPs, including mercury, and although ACI is the most widely used technology for the specific control of mercury emissions, its effectiveness is greatly reduced in the presence of SO3. Plants burning medium- to high-sulfur fuels and equipped with SCRs can have as much as 30 to 80 ppm of SO3 in the flue gas.
Research shows that even low levels of SO3 (2 to 5 ppm) can inhibit good mercury adsorption. There has been significant effort to develop sulfur-tolerant carbons to overcome this challenge, but with only limited success. Dry sorbent injection (DSI) has also been used to control SO3, but this technology is often unable to achieve the low SO3 levels required for good mercury capture, and it can adversely affect the performance of downstream particulate control equipment.
Another widely used approach for mercury control is catalytic oxidation and subsequent capture in a wet scrubber. SCR catalyst can be particularly effective for oxidation of mercury, but its performance is sensitive to flue gas temperature and halogen concentration. Wet scrubbers are very effective in capturing the oxidized form of mercury, but they will not capture mercury in its elemental form. Sometimes, oxidized mercury captured in the scrubber can be converted back to the elemental form and be “re-emitted,” thereby increasing stack emissions. Much research has been done to understand and control “re-emissions,” with some success reported in recent years. (For more on the variety of approaches to mercury capture, enter “mercury capture” in the Search box at the top of the page.)
The EPA also recently proposed new Effluent Limitation Guidelines (ELG) for the power industry that place a limit of roughly 120 parts per trillion (ppt) for mercury in wet scrubber or FGD wastewater streams. For plants that discharge wastewater from their FGD system, this may present another challenge in managing the fate of mercury with their plant. Achieving the proposed limits may require additional physical and chemical treatment of the stream to transfer the soluble mercury from the liquid phase to the solid phase, with subsequent separation and removal from the waste stream. In the worst case, expensive mercury-specific treatment techniques may be required.
In addition to mercury, these two regulations place limits on acid gas—including HCl and hydrofluoric acid (HF)—emissions in flue gas and selenium discharges in wastewater. The MATS rule requires HCl emissions to be limited to 0.002 lb/MMBtu as a surrogate for the control of emissions of acid gases. The proposed ELG rule could limit selenium levels in FGD wastewater streams to only 10 parts per billion (ppb). The ability to capture and remove these two species ahead of the wet scrubber could be advantageous and may be an effective strategy in complying with the regulations.
A New Approach to Mercury Control
Given the challenges of controlling mercury emissions, and the associated high costs, there is a need for a new cost-effective approach. Over the past decade, testing by URS and others has confirmed the significant impact of SO3 on mercury capture by activated carbon.
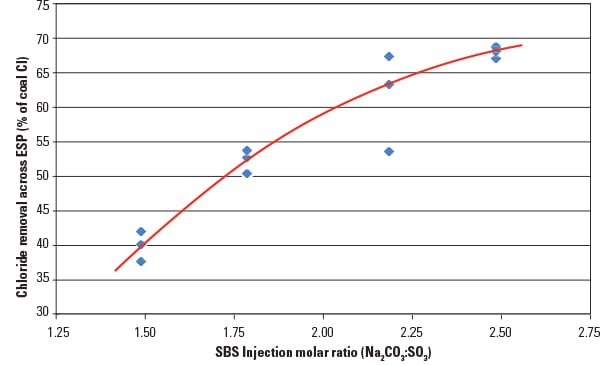
URS has gathered extensive data from existing SBS Injection installations that also show how SO3 affects the “native” capture of mercury by the unburned carbon (UBC) or loss on ignition (LOI) that is typically present in flue gas. Figure 2 shows how mercury adsorption onto unburned carbon increases dramatically as the flue gas SO3 concentration is reduced from 5 ppm to 1 ppm. The data shown were collected by varying the SBS sorbent injection rate and measuring both the mercury and LOI levels in the fly ash and the SO3 concentration in the flue gas exiting the electrostatic precipitator (ESP). The results show that reducing the SO3 down to very low levels can result in significantly higher mercury capture rates.
 |
| 2. The lower the better. Native mercury capture across the electrostatic precipitator (ESP) can be greatly enhanced when SO3 is reduced to only a few ppm. Source: URS Corp. |
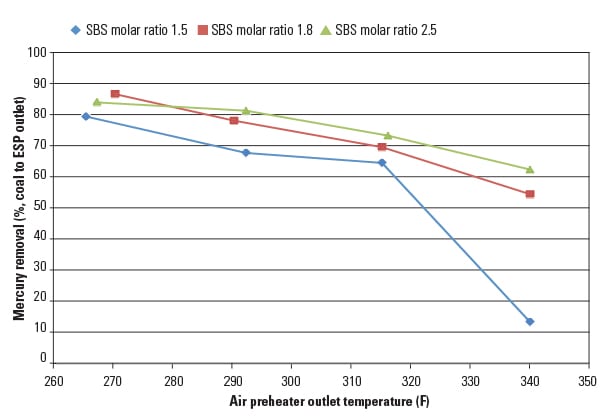
Industry research also shows that mercury adsorption onto carbon is temperature dependent with minimal capture above 350F and maximum capture below 250F. To investigate both the effect of flue gas temperature and SO3 concentration, URS recently conducted a full-scale test program with cofunding provided by the Electric Power Research Institute (EPRI) and a host utility. Testing was conducted at a Midwestern power plant with an existing SBS Injection system. The plant burns high-sulfur bituminous coal and is equipped with an SCR, ESP, and wet FGD.
During the test program, the APH exit gas temperature was varied from nominally 340F down to 290F at full-load conditions by simply varying the degree of combustion air preheat. Temperatures as low as 265F were achieved at reduced load conditions. The SBS sorbent injection rate was also varied from a typical molar injection ratio of 1.5 to an elevated ratio of 2.5 Na2CO3:SO3.
Flue gas measurements of elemental and oxidized mercury concentrations were made at the ESP outlet using a semi-continuous emission monitor and were validated with limited sorbent-trap testing in the stack. Flue gas SO3 measurements were also made at the ESP outlet using the controlled condensation sampling method. Coal and fly ash samples were collected during the test program, and both were analyzed for mercury and UBC content. Finally, HCl and selenium measurements were made in the coal, fly ash, and flue gas at the ESP outlet.
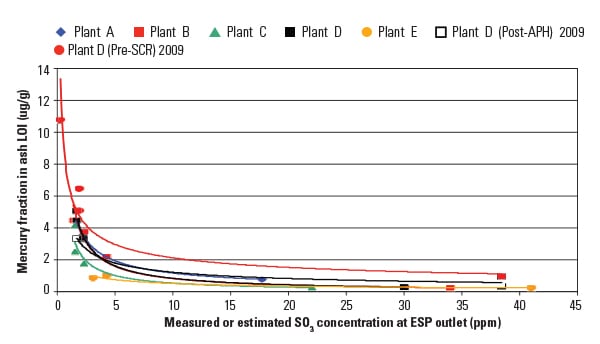
Parametric test results, summarized in Figure 3, show the overall mercury removal efficiency as measured from the coal to the ESP outlet. Results indicate that higher mercury removal was achieved by both increasing the SBS injection rate and lowering the flue gas temperature.
 |
| 3. Reducing temperature reduces emissions. Once the SO3 is removed ahead of the air preheater, mercury emissions can be further reduced by lowering the flue gas temperature. Source: URS Corp. |
At the highest temperature (340F), mercury removal was limited to about 60% at elevated sorbent injection rates. However, at the lower flue gas temperature (270F), mercury removal increased to 80% and above, even at the lowest sorbent injection rate.
Test results also indicated that both elemental and oxidized mercury were removed across the ESP, ensuring a reduction in stack mercury emissions. For example, at the lowest flue gas temperature (270F), the elemental mercury concentrations at the ESP outlet were reduced to about 0.6 lb/TBtu, well below the MATS emission limit of 1.2 lb/TBtu. During the testing, flue gas SO3 levels at the ESP outlet ranged from 0.8 to 1.2 ppm, and fly ash LOI levels varied from 3.5% to 5.0%.
Results from the test program clearly show the benefit of both reducing the flue gas SO3 concentrations to very low levels and reducing the flue gas temperature leaving the APH. Some of the approaches to lowering the flue gas temperature include eliminating preheating of the combustion air entering the APH, modifying the depth and design of the APH heating elements to increase overall heat transfer, and lowering the furnace exit gas temperature. Most plants can use one or more of these approaches to reduce their flue gas temperatures. However, Figure 3 shows that even at moderately high APH outlet temperatures, SBS Injection can enhance native mercury capture and reduce emissions.
By capturing a very significant fraction of the mercury across the ESP, the stack mercury emissions can typically be reduced to well below the MATS emission limit. Based on the positive results from the EPRI test program, the host utility has elected to upgrade its existing APH elements to achieve much lower flue gas temperatures and much higher mercury capture—a key component of its MATS compliance strategy.
Other power plants are also exploring how they can use their SBS Injection system to reduce mercury emissions. Recent testing at an SBS installation in West Virginia has also shown that reducing the APH operating temperature can lower mercury emissions. In the Midwest, Indianapolis Power & Light recently selected the SBS Injection process for its Petersburg Station specifically to control both SO3 and mercury as part of its MATS compliance strategy. For plants burning fuels with a range of sulfur and mercury levels, the SBS injection process can effectively mitigate the impact of SO3, thereby maximizing the native capture of mercury and minimizing emissions.
Furthermore, by reducing the SO3 concentration at the inlet to the APH to very low levels, it is also possible to eliminate fouling of the APH due to sulfuric acid and/or ammonium bisulfate. Based on research and testing conducted by a leading APH manufacturer, the APH can now be reliably operated at much lower temperatures, improving mercury capture and plant heat rate or energy efficiency. One might say that this new approach allows a power plant to “capture more mercury … by burning less coal.”
Capturing more mercury in the fly ash also significantly reduces the amount of mercury that is captured by the wet scrubber, which can provide several potential benefits. First, if less mercury is retained in the scrubber, then the potential amount of mercury that can be “re-emitted” is also reduced, thereby lowering the risk that this phenomenon will result in exceedences of the MATS limit. Second, by lowering the mercury content in the FGD scrubbing liquor, it is much more likely that ELG limits for mercury content in FGD wastewater streams can be met without the need for additional treatment.
HCl and Selenium Co-Removal
As mentioned earlier, HCl emissions in flue gas and selenium discharges in wastewater are also regulated by MATS and proposed ELG rules, respectively. Recent testing has shown that sorbent injection for the removal of SO3 and mercury is also effective for the capture and removal of HCl and selenium from flue gas.
HCl removal results as a function of the SBS sorbent injection rate are illustrated in Figure 4. At typical SBS sorbent injection rates, roughly 40% HCl capture was achieved, while elevated injection rates resulted in nearly 70% HCl capture. These results are consistent with previous testing that shows that SO3 is preferentially removed, with excess sorbent available to remove HCl present in the flue gas. However, results at a given plant will vary depending on the relative concentrations of SO3 and HCl in the flue gas, and the overall sorbent injection rate.
Co-removal of HCl with the fly ash can provide several advantages to the operating plant. Some plants must control the dissolved chloride levels in their wet scrubbers due to materials of construction and corresponding corrosion concerns. As a result, many plants must operate with a chloride purge stream from the FGD system. In most cases, this chloride purge stream must be treated prior to discharge.
In addition, the newly proposed ELG rules may require additional treatment to meet new stringent limits for mercury and selenium. By capturing HCl in the fly ash, and reducing the amount captured in the FGD system, it may be possible to greatly reduce, or even eliminate, the need for a chloride purge stream. As a result, it may be possible to avoid the significant capital and operating costs associated with FGD wastewater treatment.
Effective capture of selenium has also been demonstrated using the SBS Injection process. Figure 5 shows selenium removal across the ESP as a function of the SBS sorbent injection rate and flue gas temperature at the APH outlet. Results indicate that 60% to 90% capture efficiency was achieved over the range of sorbent injection rates tested. As described earlier, the newly proposed ELG rules place stringent limits on selenium in FGD wastewater streams. By capturing a significant fraction of the gaseous selenium with the fly ash, it may be possible to meet the ELG limits without the need for costly treatment technologies.
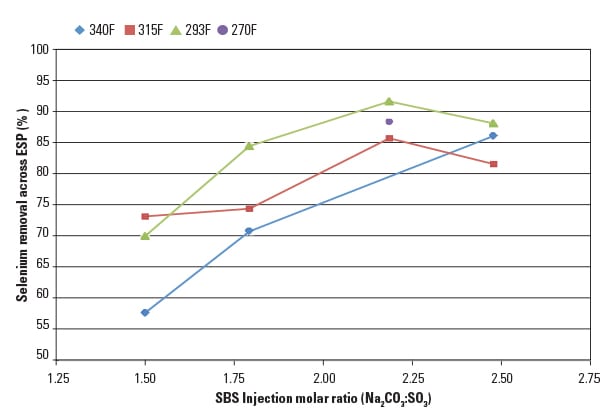 |
| 5. Captures selenium too. Significant co-removal of selenium across the ESP using SBS Injection can help plants meet the stringent proposed effluent limitation guidelines. Source: URS Corp. |
It All Adds Up
Achieving new stringent MATS and ELG limits presents numerous challenges for conventional approaches that will require significant capital expenditures, as well as increased plant operating costs. As described here, recent testing has shown that the injection of a single sorbent can effectively remove the regulated pollutants while improving plant heat rate and energy efficiency, reducing plant operating costs, and achieving significant co-removal of HCl and selenium—which may make it possible to avoid costly wastewater treatment requirements resulting from the proposed ELG rules. ■
— Sterling Gray ([email protected]) is a technology and business development manager in the Process Technologies group at URS Corp. Jim B. Jarvis is a senior project manager, and Steven W. Kosler is a senior process engineer for URS Corp.