Biofouling control options for cooling systems
The typical multi-plant utility spends millions of dollars a year on bleach, bromide, and other biocides to keep heat-exchange surfaces clean and to control biofouling in cooling systems. Proper use of this style of chemical warfare begins with understanding the enemy.
Biofouling can be either micro or macro (by clams or mussels) in nature. The first half of this article explores microfouling by bacteria, slimes, algae, and the like. The second half discusses macrofouling by clams or mussels; their control is a specialized water-treatment discipline.
Part I: Microfouling
Microbiological fouling in cooling water is caused by biofilms (Figure 1). The biofilms are loose configurations of many different kinds of bacteria; a slimy substance they produce called exopolysaccaride (EPS) holds it all together. Biofilms, which cause dental cavities and diseases such as cystic fibrosis, are ubiquitous in virtually all kinds of water piping. In industrial processes, biofilms can cause corrosion and seriously affect flow, pressure drop, and the efficiency of heat transfer from metal surfaces (Figure 2).
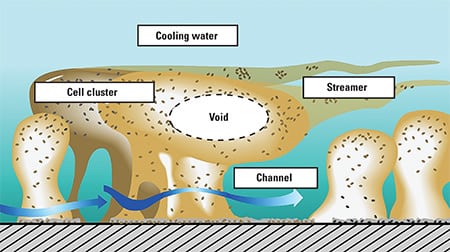
1. Some need oxygen; others don’t. Biofilms live in a variety of environments, from aerobic, in cooling water (top), to anaerobic, near metal pipe surfaces (bottom). Source: P. Dirckx, MSU Center for Biofilm Engineering, Montana State University
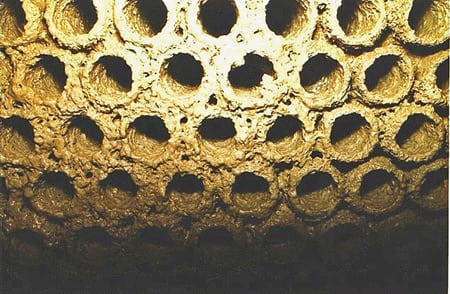
2. Can’t stay clean. Biofouling limits heat transfer from condenser tubing. Courtesy: K.A. Selby
Microbiologically influenced corrosion (MIC) is often a consequence of microbiological fouling. To cause corrosion, a biofilm creates cathodic and anodic areas on the metal surface. The bacteria in it can produce acids that are corrosive to metals and may even use a metal as fuel. (For details, see James Walker, Susanne Surman, and Jana Jass, "Industrial Biofouling: Detection, Prevention, and Control" [John Wiley & Sons Inc.: 2000], p. 86.)
Biofilms also serve as collection sites for silt and other debris that can accumulate on cooling tower fill and lead to structural damage. They encourage the formation of scales such as calcium carbonate. Biofilms also can harbor populations of disease-causing bacteria such as Legionella and listeria.
The general rate of biofilm growth is controlled by nutrients and the ambient conditions of the cooling water. Growth is slower in winter, when temperatures are cooler. The type of water source also is important. The increasing use of industrial and municipal effluent as makeup water for cooling systems is great news for the bugs, because these waters often contain significant amounts of the nitrogen, phosphate, and organic compounds necessary for their growth.
Invisible invaders. A bacteria-free cooling water system (any water-containing system, in fact) isn’t to be found in the power industry. The sources of bacteria are just too numerous, and bacteria’s adaptability to conditions allows them to set up shop just about anywhere.
The bacteria that inhabit a cooling system typically begin their lives as free-swimming or planktonic bacteria. They can be introduced from the raw water source or as spores from the air. Although there may be billions of planktonic bacteria in a cooling system, they are typically not a problem. As long as they keep moving, they cannot cause corrosion or heat transfer issues. And they can be easily destroyed by even small concentrations of any number of oxidizing and nonoxidizing biocides.
The bacteria only become problematic when they settle down and become part of an established biofilm. Unfortunately, most techniques used by the industry and vendors of biocide chemicals to gauge the number and/or type of bacteria in a cooling system detect only planktonic bacteria. The number of planktonic bacteria and the prevalence of harmful biofilms in a cooling water system are not well correlated.
Sticky wicket. At some point, using mechanisms that are not completely understood, free-swimming bacteria attach themselves to surfaces and form colonies. To form a biofilm, the bacteria excrete EPS and encase themselves in it. Although it is almost entirely water, EPS is extremely effective at protecting the bacteria from changing conditions in the outside world. EPS also excels at catching nutrients, suspended debris, and other bacteria, which improves the biofilm’s resilience and durability.
As the biofilms form, they incorporate and support many different types of bacteria. While bulk cooling water and the top layer of a biofilm may represent an aerobic (oxygen-rich) environment, in lower layers (adjacent to piping walls) the environment is anaerobic (oxygen-deficient). Different kinds of bacteria exploit the conditions in the biofilm that best suit them. If a biofilm spreads, so do the bacterial colonies. Bacteria on the portion of biofilm in the cooling water can be sloughed off and settle elsewhere to form more biofilms throughout the system.
Bacteria in the oxygen-rich outer layers are more active than those in the inner layers, which appear relatively dormant. Interestingly, studies show that that there are far more dormant bacteria in the interior of the biofilm than near its surface.
These characteristics of biofilm structure suggest how difficult it can be to control or eliminate established biofilms using biocides alone. Bacteria in biofilms have proven 10 to 1,000 times more resistant to biocides of any type than their planktonic counterparts. Biocides can only kill bacteria they come into contact with.
A biofilm has relatively few bacteria in its outer, metabolically active areas that biocides touch. Oxidizing biocides, such as bleach, are indiscriminant: they can be entirely depleted as they burn through the outer layer and EPS of the biofilm, never reaching the highest concentrations of bacteria. That’s why the infrequent application of even "shock" dosages of bleach is ineffective at controlling biofilms. Furthermore, because the bacteria on the inside of the biofilm are mostly dormant, biocides that act as metabolic poisons may not completely destroy a biofilm.
Dispersants may be used in concert with biocides to lower the local concentration of EPS, thereby increasing the penetration of biocides into the biofilms and the destruction of the bacteria they harbor. However, the best approach is to prevent biofilms from ever getting so well established that they can bounce back. As one biofilm expert is fond of saying, "It’s the same thing as your mother told you about your bedroom—it’s much easier to keep it clean than it is to make it clean in the first place."
Take out the trash. What no biofilm can defend against is mechanical removal. Whether they use brushes, scrapers, or foam balls, mechanical systems are very effective at removing biofilms from heat-exchange surfaces and dispersing them into cooling water. In recirculating systems such as cooling towers, it is very important to couple a mechanical cleaning with an application of biocides and perhaps biodispersants. Although mechanical removal doesn’t kill the bacteria, it is very effective at disrupting the structure of the biofilm, making all the bacteria in it more vulnerable to biocides.
Biofilms and biofouling can occur on nearly every material. In fact, some alloys normally considered vulnerable to general corrosion may be less susceptible to biofouling than alloys advertised as "corrosion-resistant."
MIC can attack the stainless steel used in many cooling water systems in any of several ways. Once a biofilm has broken through the protective oxide coating, corrosion can proceed rapidly. The anaerobic conditions at the base of the biofilm prevent the protective oxide layer from reforming (Figure 3). The corrosion can be very localized and undercut in appearance. In some cases, the corrosion "tunnels" underneath the metal (Figure 4).

3. Urban blight. Bacteria from New York City’s East River were responsible for this severe pitting of 316L stainless steel in a power plant. Courtesy: M&M Engineering

4. Hungry critters. Biofilms in stagnant well water caused corrosion in 304L stainless steel adjacent to a weld. "Tunneling" commonly accompanies microbiologically induced corrosion. Courtesy: M&M Engineering
Even if the metal doesn’t corrode, the inert nature of some alloys (those with titanium, for example) makes the metal a very suitable host for a biofilm. Copper alloys, by contrast, tend to actively prevent biofilm growth. Corrosion on the surface of copper piping liberates copper ions that can act as a biocide, poisoning bacterial growth. Copper can have the same effect on macrofouling, by preventing the attachment of clams and mussels.
Fighting fouling. Chemical biocides can be divided into two major groups, oxidizing and nonoxidizing. Oxidizing biocides include chlorine (gas and bleach), hypobromous acid, BCDMH (1-bromo, 3-chloro, 2,2-dimethyl hydantoin), chlorine dioxide, hydrogen peroxide, and ozone. Common nonoxidizing biocides include gluteraldehyde, isothiazalone, and DBNPA (2,2-dibromo, 3-nitrilopropionamide).
Chlorine used to be power plants’ most commonly used biocide. Recently, many U.S. plants have forsaken their less-costly gaseous chlorine systems in favor of more-expensive liquid bleach. Regulatory and safety issues associated with the transportation, storage, and use of 1-ton chlorine cylinders made their continued use too risky and uneconomic.
When chlorine gas is added to water it reacts to create hydrochloric acid and hypochlorous acid in equal proportions. In a recirculating cooling water system, the application of chlorine gas often was accompanied by a drop in pH.
Cooling systems that use chlorine effectively often operate at a pH close to 7. At this level, chlorine and hypochlorous acid are very effective. It is the hypochlorous acid (HClO) that serves as the biocide, by penetrating the cell membrane and disturbing several vital cell mechanisms. However, in alkaline environments hypochlorous acid is neutralized and forms hypochlorite ions (ClO-1). In such environments, the ions haven’t proven capable of serving as a biocide, probably because they cannot penetrate the cell walls of bacteria as easily as HClO can.
Bleached blond. Commercial or industrial bleach is available in strengths from 10% to 15%. It is manufactured by bubbling chlorine gas through a sodium hydroxide solution. Excess hydroxide is maintained in the final solution to increase the stability of the bleach (the strength of bleach decreases with time, exposure to UV light, and contact with a number of metals). Typically, commercial bleach has a pH between 11 and 13. At these highly alkaline levels, the bleach contains no hypochlorous acid, only hypochlorite ions. One possible consequence of the high pH is the formation of calcium carbonate deposits on bleach injectors, the result of the bleach reacting with "hard" cooling water (Figure 5).

5. Big deposit, lower return. The high pH of bleach caused the calcium carbonate deposits that are plugging this bleach injection line. Courtesy: M&M Engineering
Unlike dosing with chlorine, adding bleach to cooling water either slightly increases its pH or has no effect on it. If the water is already alkaline, more ineffective hypochlorite ions will be formed, to the detriment of creating more-effective hypochlorous acid. In pH 8 cooling water, the concentration of HClO is 20%, compared to 70% in water with a pH of 7. Many of today’s recirculating cooling systems operate at a pH between 8.0 and 8.5. Lake water used for cooling can be quite alkaline.
Besides acting as a biocide, chlorine or bleach can react with any number of other organic and inorganic compounds. If the cooling water contains amines or ammonia, as many cooling waters do, chloroamines are formed. Because the chloroamines consume chlorine, they increase the amount of chlorine needed to produce a specified free-chlorine residual level. Chloroamines also can act as biocides, but not very effectively.
When measuring free available chlorine, it’s important to remember that the reagents added for a DPD (4,5-dihydroxy, 2,3-pentanedione) test will measure the concentrations of both hypochlorous acid and hypochlorite ions—as well as those of other oxidizing species such as hypobromous acid, hypobromite ions, and peroxide. Therefore, if a measurement of cooling water with a pH of 8.5 indicates that it has 1 ppm of free available chlorine, its concentration of bacteria-killing hypochlorous acid will be only about one-tenth of that level, or 0.1 ppm. The other 0.9 ppm represents hypochlorite ions.
The homebrew approach. You need not purchase commercial or industrial bleach in bulk to obtain hypochlorite. Many seaside power plants produce their own hypo by feeding seawater to an electrically powered generator that produces a more dilute hyprochlorite solution than a commercial bleach, but because of its freshness, some claim that it is more effective against biofilms. MIOX Corp. (www.miox.com) uses a proprietary process to generate a "mixed-oxide" biocide containing chlor-oxygen compounds that the company claims are more effective against biofilms than bleach alone.
Given the ineffectiveness of bleach in alkaline environments, many utilities that have alkaline cooling water have switched to the use of hypobromous acid (HBrO). Hypobromous acid is like hypochlorous acid in this regard: The acid form is an effective biocide, whereas hypobromite ions (BrO–) are not. However, hypobromous acid remains in the acid form at higher pHs than does hypochlorous acid (Figure 6) and therefore is many times more effective for treating cooling water with a high pH. Bromoamines, unlike their chlorinated counterparts, appear to be very effective biocides. For this reason, hypobromous acid is often preferred for treating cooling water (and wastewater) that contains ammonia.

6. Bugs, beware. The pH of cooling water affects the effectiveness of biocides. The graph shows its effect on the ionization of hypochlorous (HClO) and hypobromous (HBrO) acids. Source: M&M Engineering
The only fly in this ointment is the instability of hypobromous acid at near-neutral pHs. In a neutral or slightly alkaline environment, it decomposes into bromate (BrO3-), a carcinogen and neurotoxin. Accordingly, the amount of bromate formed may be of concern to plants that discharge their cooling water into a river or lake. In very acidic environments, hypobromous acid is relatively stable.
Hypobromous acid can be purchased, or produced on-site. Nalco Co. (www.nalco.com) sells hypobromous acid that has been stabilized with a reducing inorganic acid under the trade name Stabrex. Hypobromous acid also can be generated off-site from solid hydantoin products such as BCDMH. This chemical is often found under its tradename, Towerbrom, from Occidental Petroleum Corp. (www.oxy.com).
More commonly, however, hypobromous acid is generated on-site by reacting hypochlorous acid with a sodium bromide solution. The latter is typically shipped at a concentration between 38% and 46%. Solutions above 40% are more susceptible to freezing. Sodium bromide is mined in Arkansas and Michigan, and the two states account for nearly all U.S. production of the chemical. There are also sources of sodium bromide in the Middle East.
Because it typically takes a lot of sodium bromide to treat cooling water, its cost may be a significant chemical expense for the plant. However, many plants are not properly using it and are thus wasting their money.
Although sodium bromide is delivered with very specific instructions on how to properly blend it with bleach to achieve full activation, a number of power plants either not seen the instructions or have been told by their supplier not to bother following them. Some plants simply mix the concentrated chemicals and inject the solution into cooling water. Others add the bleach and bromide using separate lines. Both methods are inefficient and waste a significant amount of the bromide.
The seminal work on bromide reaction chemistry was published in 1987 by K. Kumar and D.W. Margerum at Purdue University ("Kinetics and Mechanism of General-Acid-Assisted Oxidation of Bromide by Hypochlorite and Hypochlorous Acid," Journal of Inorganic Chemistry, pp. 2,706–2,2711, [www.chemistry.org].) Their research found that hypobromous acid was formed by reacting bromide with hypochlorous acid. The reaction was fast and complete if the hypochlorite solution was neutral, acidic, or slightly alkaline. However, if the hypochlorite solution was strongly alkaline (as would be the case for a concentrated commercial bleach solution), the reaction proceeded at a considerably slower rate. Kumar and Margerum reported that the reaction rate for hypochlorous acid was 1.5 million times faster than for hypochlorite ions. At a pH of 12, the bleach and bromide must be in contact for more than 45 minutes to convert just 50% of the bromide to hypobromite.
Optimizing the biocide feed. In biocide addition systems, the concentrated bleach and sodium bromide solutions are typically only in contact for a matter of seconds before the cooling water dilutes them to the point where they can no longer effectively react. If applied in this way, it is likely that only a small percentage of the bromide is actually converted to hypobromous acid. That many plants get positive results from adding bromide suggests the effectiveness of even small amounts of hypobromous acid as a biocide.
To maximize the effectiveness of sodium bromide, solutions containing it should be injected into a sidestream of cooling water and blended thoroughly in an in-line static mixer. Then, the bleach should be added and the solution mixed again. The concentrations of bromide and bleach are often added on an equimolar basis. The flow of dilution water flow should be set to produce a final oxidant concentration between 1,000 ppm and 2,000 ppm. Your sodium bromide supplier should be able to provide a product application guide containing equations that allow you to optimize the configuration of your bleach/bromide addition system.
Peroxide: the "green" choice. Another oxidizing biocide that has been used successfully in plant cooling water systems is hydrogen peroxide, a very strong oxidant that destroys bacteria and biofilms. Peroxide has the distinct environmental advantage of producing only oxygen as a byproduct of its reaction.
Much of the corrosion damage done by biofilms is in the anaerobic area at their base. Sulfate-reducing bacteria (SRBs) thrive in these anaerobic conditions and produce corrosive acids. Peroxide doesn’t just "burn" through the biofilm; it permeates it with oxygen, destroying the SRBs.
Hydrogen peroxide worked well at one plant whose cooling tower fill had been so fouled by accumulation of biofilms and debris that the tower’s structure was strained to the breaking point (Figure 7). Repeated injections of industrial-strength hydrogen peroxide into the tower’s cell riser eliminated the films and the debris that they attracted.

7. Tower cleaner. The biofilms on this cooling tower fill were effectively controlled with hydrogen peroxide. Courtesy: M&M Engineering
Our company has developed on-site hydrogen peroxide generators that requires only air and power. Field tests are being planned for this equipment to test its effectiveness. On-site generation of hydrogen peroxide, of course, would eliminate transportation costs and storage issues.
Part II: Macrofouling
Macrofouling occurs when debris large enough to interfere with flow (leaves, sticks, plastic bags, etc.) finds its way into a cooling water system. Biological organisms in seawater (barnacles, bivalves, seaweed, etc.) and freshwater (fish and algae) also qualify as macrofoulants. Most seawater cooling system designs include mechanisms for removing traditional macrofoulants at the source.
For U.S. designers of freshwater cooling (and fire protection) systems, however, keeping the system free of macrofoulants is an uphill battle. Their archenemies are Asiatic clams and zebra and quagga mussels, invasive species that may not have made the scene when the plant was built.
Asiatic clams. Corbicula fluminea have been in the U.S. the longest of the three species. They began arriving on the West Coast from Southeast Asia in the 1920s. By the 1970s, they had become strongly established in the Midwest. By the 1980s, they had reached the East Coast. Figure 8 shows their current distribution.

8. Eighty years of migration. The current distribution of Asiatic clams in the U.S. Courtesy: U.S. Geological Survey
The Asiatic clam is a bivalve that can be as large as 1.5 inches across, although most are a bit smaller. At that size, they fit snugly into most heat exchanger tubes and fire protection piping, where they enjoy the flow of water—until they cause it to stop. That’s exactly what happened at a nuclear plant in Arkansas in 1980, and the event triggered mandatory inspections of all such plants in the U.S. Today, most freshwater-cooled American power plants (both nuclear and fossil-fueled) run ongoing programs to control infestations of Asiatic clams.
Asiatic clams begin reproducing when in the spring, when the water temperature rises to about 60F. Reproduction continues unless the water temperature is above 86F. During the spawning period, a single hermaphroditic (both sexes in the same animal) adult clam can produce hundreds or thousands of larvae per day.
Zebra mussels. Dreissena polymorpha are a newer arrival. They were first found in Lake St. Clair (between Detroit and Windsor, Ontario) in 1988. Figures 9 and 10 show the distributions of zebra mussels in the U.S. in 1988 and 20005, respectively. America’s first zebra mussels were international stowaways in the ballast water of cargo ships from the Black Sea. After the ships made their way down the St. Lawrence Seaway, the mussels were discharged along with the ballast water.
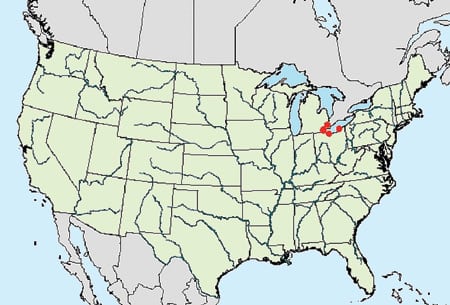
9. Then . . . Zebra mussel distribution in the U.S. in 1988. Courtesy: U.S. Geological Survey

10. . . . and now. Zebra mussel distribution in the U.S. in 2005. The stars indicate isolated waterways and cooling systems where mussels have been found recently. Courtesy: U.S. Geological Survey
The maritime shipping industry has tried to prevent zebra mussels from becoming established west of the Continental Divide by stepping up inspections of ships’ bottoms, boat cleaning, and other methods. To date, these efforts have been only partially successful. The yellow stars in Figure 10 pinpoint where zebra mussels have recently been found, both east and west of the Mississippi.
Zebra mussels have a lifespan of two to five years and are slightly larger than Asiatic clams—up to 2 inches across. At that size, they can completely block water intake structures on lakes and rivers. Zebra mussels attach to the structures (and to each other, forming colonies) by means of byssal threads. Colonies have been reported achieving densities of 750,000 to 1,000,000 mussels per square meter. Figure 11 shows a particularly bad infestation.

11. Submarine attack. Zebra mussels blanketing an underwater structure at a power plant. Courtesy: M&M Engineering
Zebra mussels are filter feeders and remove plankton and other small organisms from the water. Zebra mussels in the Great Lakes compete with native species for the food supply. Another environmental impact has been an increase in water clarity. Although this may seem like a good thing, the increased water clarity means that sunlight penetrates deeper and accelerates the growth of algae. Among other things, this has led to taste and odor problems in the Great Lakes. Power plant and municipal intake structures have been fouled by some of these algae (e.g., Cladophora). This has caused blocked flow and reductions in plant output.
Quagga mussels. Dreissena bugensis, close relatives of the zebra mussels, are the most recent biological macrofoulant arrival. They were first detected in the U.S. in 1989. According to one source, the density of quagga mussels on the bottom of Lake Michigan increased from 899 per square meter in 2000 to 7,790 per square meter in 2005. By January 2007, quagga mussels had been discovered in Lake Mead, Lake Mohave, and Lake Havasu (Figure 12).

12. Go west, young mussel. Quagga mussels’ current distribution in the U.S. The red dots indicate recent outbreaks. Courtesy: U.S. Geological Survey
Quagga mussels cause most of the same problems as zebra mussels, but they have an even greater negative environmental impact. Quagga mussels are active at lower temperatures than zebra mussels and thus inhabit deeper areas of a lake. Locating an intake pipe as deep as 500 ft below the surface may not keep quagga mussels out of it. Like zebra mussels, quagga mussels are filter feeders, but they are even bigger eaters. In fact, quagga mussels have almost completely displaced zebra mussels in many parts of Lake Michigan.
The life cycles of Asiatic clams, zebra mussels, and quagga mussels are similar. During their early larval and veliger stages, they are all planktonic (disbursed in the water column). During later stages, the veligers (now equipped with a velum to help them swim and feed) begin to settle on surfaces as they grow to adulthood.
War on underwater terror. Strategies for controlling Asiatic clams, zebra mussels, and quagga mussels are likewise similar. They include physical removal, applying a metallic coating or polymer to the interior of pipes and the exterior of structures, heating piping and structures, and—the subject of this article—the use of oxidizing and nonoxidizing biocides.
Some control strategies target the clams and mussels when they are vulnerable larvae and veligers. Others don’t try to kill the organisms, but rather prevent them from settling down as veligers. Still others focus on killing or removing adults.
Methods for physically removing mollusks (clams, mussels, and other bivalves) may be as simple as scraping or dredging the population from impacted areas. Some processes require draining structures; others are conducted underwater using equipment or divers.
Coatings have long been used to reduce the accumulation of marine organisms on boat hulls and other underwater structures. Most coatings contain a metal (copper or tin) or another substance (such as a polymer) that makes it harder for larva/veliger to cling to the surface.
Thermal treatment has been used successfully to control zebra mussels because they are essentially cold-water creatures. Although their maximum survival temperature depends on several factors, water hotter than 90F is usually fatal to zebra mussels if they are exposed to it long enough. Temperatures exceeding 95F produce rapid mortality. Quagga mussels are even less tolerant of heat; 86F water is enough to kill even the hardiest adult. By contrast, Asiatic clams are much more heat-tolerant than zebra or quagga mussels, so thermal treatment for Asiatic clam control usually doesn’t work.
Oxidizing biocides such as chlorine gas, sodium hypochlorite (bleach), chlorine dioxide, or potassium permanganate can be used to kill both veligers and adult mollusks. Veligers can be controlled with intermittent doses, but killing adults takes about two weeks of continuous feed of an oxidant; the adults can literally "clam up" for that long to avoid it. Some studies have shown that a pulsed-feed approach (with alternating on and off periods) works just as well. Finally, it’s worth mentioning that using chlorine dioxide to kill adult clams or mussels requires less time (about six days) to be effective.
Nonoxidizing biocides, such as certain quaternary ammonium compounds, are very effective molluscicides. They can kill off an entire population within 48 hours. However, their use usually requires a detoxification step, such as adsorption with bentonite clay, before discharge. These biocides, which have a strong cationic (positive) charge, interfere with mollusk respiration. So do some conventional water treatment polymers (dispersants and flocculants), which eventually clog the gills of mollusks if applied at elevated dosages.
—David Daniels ([email protected]) is a senior consulting scientist for M&M Engineering and a POWER contributing editor. Tony Selby ([email protected]) is a principal scientist for M&M Engineering.