Big batteries blooming
All interconnected transmission and distribution (T&D) grids have one thing in common: Their operators must continually dispatch generators to keep the network’s supply and demand in balance at all times and to maintain its voltage and frequency within very tight tolerances. Threatening to change this paradigm by adding a new variable—bulk electricity storage capacity—to the balancing equation are new and rechargeable sodium-sulfur, vanadium redox, and zinc-bromide batteries. Some of these—as well as nickel-cadmium batteries, which went commercial decades ago—have already demonstrated their ability to act as generators as large as 10 MW.
These batteries may play a role in addressing a common set of problems that T&D system planners worldwide face:
- In many parts of the world, investment in the carrying capacity of T&D systems has failed to keep pace with load growth. This oversight has increased constraints on already overtaxed systems and reduced their tolerance for faults, as the August 2003 blackout of much of the eastern U.S. and southern Ontario demonstrated.
- In the wake of the terrorist attacks of September 2001, awareness of the vulnerability of the electricity supply system to intentional disruption has raised interest in the distributed grid concept. A distributed grid has many small and geographically dispersed energy resources sited close to load centers, in contrast to a centralized grid, which draws on a few large generators far from load centers.
- The growth of the Internet and the broad and growing penetration of electronic controls for commercial and industrial processes have created a need for improved power quality and reliability. Even very brief excursions from nominal power supply voltages can cause electronic controllers to shut processes down, which can result in extended downtime, damaged equipment, and scrapped product.
The ongoing dissolution of the traditional electricity sector structure also seems to call for increased reliance on big batteries wherever feasible. According to Jason Makansi, executive director of the Energy Storage Council, one consequence of deregulation is that in many states, generation and T&D are no longer planned in an integrated fashion by one entity—the local utility. "Storage in general, and batteries in particular, can help manage the gaps created by the disaggregation of the electricity production and delivery value chain," Makansi explains.
Storage also has a critical role to play in securing the nation’s energy infrastructure, much as the Strategic Petroleum Reserve does for oil, and bulk gas storage does for balancing seasonal natural gas demand and supply.
If you’re responsible for T&D system planning and are ready to begin considering energy storage a viable option, the table and the accompanying box are a good place to start. The table summarizes the status of and future prospects for several battery technologies that either currently offer bulk energy storage capability or promise to do so in the near future. The following discussions and case studies explain how the new bulk-storage batteries work and how pioneers are using them to solve a number of common T&D problems.
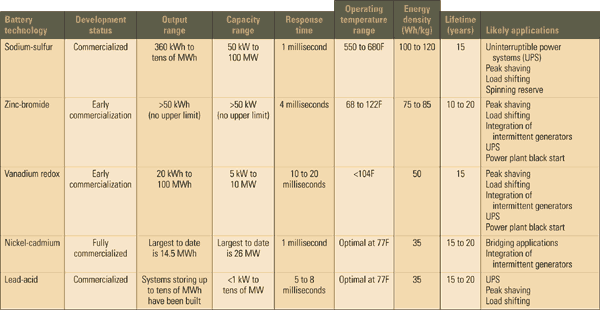
A comparison of today’s bulk electricity storage technologies
Source: Platts
Sodium-sulfur: A hot new battery
Ford Motor Co. (Auburn Hills, Mich.) is credited with launching development of the sodium-sulfur (NaS) battery in the 1960s for vehicular applications. However, today’s next-generation NaS batteries are the end result of more than a decade of R&D by NGK Insulators Ltd. (Nagoya, Japan) and Tokyo Electric Power Co. (Tepco). Tepco had identified NaS technology as the best candidate for replacing central pumped-hydro energy storage in Japan. NGK, which supplied the expertise in ceramics that was crucial to development of the technology, is today the only supplier of NaS batteries for bulk storage applications.
Sodium-sulfur batteries use molten sulfur as the positive electrode and molten sodium as the negative electrode. These active materials are separated by a solid, ceramic electrolyte that conducts sodium ions (Figure 1).
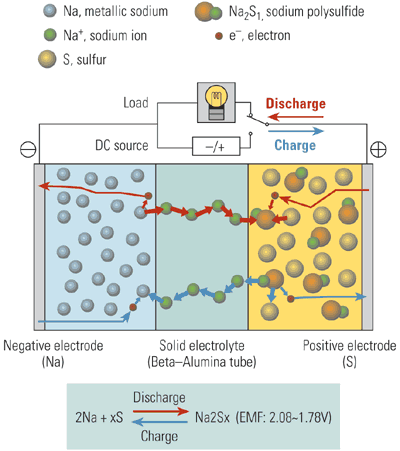
1. Sodium-sulfur (NaS) battery operation. A solid ceramic electrolyte separates the liquid sulfur positive electrode from the liquid sodium negative electrode.
Source: NGK Insulators Ltd.
During discharge, positive sodium ions flow through the electrolyte to combine with the sulfur, forming sodium polysulfides. Electrons flow through the external circuit of the battery to create a potential difference. Charging of the battery releases positive sodium ions from the sodium polysulfides, sending them back through the electrolyte to recombine as elemental sodium. To keep the electrodes in the molten state, the battery is kept at a temperature of about 570F. NaS modules also include an electric heater to keep the reactants molten and thermal insulation to reduce heat losses.
NGK’s new, commercial-scale NaS battery production facility—which opened in April 2003 in Komaki, Japan—has an initial capacity of 65 MW/yr that can be expanded to 200 MW/yr. Modules are currently available with ratings of 50 kW/360 kWh or 430 kWh for peak-shaving (PS) applications and 50 kW/360 kWh or up to 250 kW for short-term power quality (PQ) applications. Units can be combined to provide up to 20 MW for PS and up to 100 MW for PQ applications. PS modules are optimized to deliver long discharges with modest voltage drop, whereas PQ modules are designed to deliver short pulses of power.
A precommercial version of the NaS battery has been demonstrated at about 90 sites in Japan since April 2004. One system installed at a Japanese semiconductor factory was rated at 1 MW/7 MWh for peak shaving duty and supplied up to 3 MW for 13.5 seconds to improve power quality. The system also proved its ability to lower the factory’s cost of energy by shifting some purchases from on-peak to off-peak hours, and to protect important loads from short outages. Another of its benefits was leveling the local utility’s demand.
In the only U.S. demo of the NaS battery to date, a system was installed in September 2002 at an American Electric Power Co. office building in Gahanna, Ohio. It can simultaneously provide 500 kW for up to 30 seconds for PQ needs and 158 kWh at up to 100 kW for peak shaving. Alternatively, the system can provide 300 kW for 30 seconds for power quality and 720 kWh at up to 100 kW for peak shaving.
Compared to conventional lead-acid batteries, NaS batteries offer three to five times the energy density and more and deeper discharge cycles. The units are easily sited indoors or outdoors, as their operation is clean, quiet, vibration-free, and insensitive to ambient temperature. Their major downside is their capital cost; where lead-acid batteries run about $400 to $900/kW, the current NaS modules—which are nominally rated at 50 kW each—are priced at $1,800/kW.
Flow batteries’ pros and cons
Flow batteries (FBs) are a relatively new class of electrochemical device. They can store large amounts of electrical energy (from tens of kWh to tens of MWh) and deliver it either slowly over several hours or within milliseconds or minutes as high-power pulses. The versatility of FBs equips them for a wide range of applications—from energy management to power quality and reliability.
There are three different types of flow batteries, and they use different chemicals to store energy. Two, which use zinc-bromide and vanadium electrolytes, are currently in the early stages of commercialization. Until recently, the third type—the Regenesys battery, which uses sodium-bromide and sodium-polysulfide electrolytes—seemed to have a bright future. But a year ago the German utility giant RWE, Regenesys Technology Ltd.’s corporate parent, abruptly pulled the plug on the R&D effort. Later, RWE sold its intellectual property rights to the technology to Vancouver-based VRB Power Systems Inc., so the Regenesys battery may yet be resurrected.
Flow batteries will have—at least initially—higher capital costs than lead-acid batteries (typically, $350/kWh vs. $200/kWh for a load-shifting application, excluding power conditioning and balance-of-plant costs), but they also have a much longer cycle life. Accordingly, zinc-bromide and vanadium batteries may be economically attractive to certain users for certain applications. And their cost is certain to fall as production ramps up to commercial levels within the next few years and the economies of scale kick in.
The big operational advantage of flow batteries, which use reversible reduction-oxidation (redox) reactions, is that they allow either power or energy output to be optimized in real time. In a conventional battery, both electrodes are immersed in the same ion-conductive electrolytic solution. During discharge, oxidation at the battery’s negative electrode (anode) liberates electrons. The electrons then leave the anode, flow through the external circuit to which the battery is connected and perform useful work, and are returned to the positive electrode (cathode). At the cathode, reduction recombines the electrons with positive ions in the electrolyte.
The process is similar in the flow battery, except that each electrode is immersed in a different electrolyte, and the two electrolytes are separated by an ion-exchange membrane. To discharge electricity, the two electrolyte solutions are pumped from separate tanks into half-cells along either side of the membrane (Figure 2). To store electricity, current is put back into the system to return the electrolytes to their original chemical states, recharging the battery.
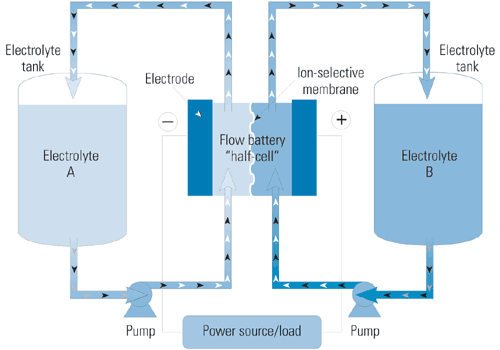
2. Representative flow battery design. Flow batteries store electrical energy chemically in tanks separated from the electrochemical cells. Each tank contains a different electrolyte solution. Electrolytes are pumped through half-cells, exchanging ions across selective membranes during charging and discharging, producing or absorbing electrons.
Source: Platts; adapted from Regenesys Technologies Ltd.
NiCad batteries: Less costly than they seem
Nickel-cadmium (NiCad) batteries have powered portable electronic devices for decades. However, their relatively high capital cost has prevented their widespread use in large stationary applications. Nevertheless, there are certain utility market niches in which NiCads can compete on a lifecycle-cost basis with conventional lead-acid batteries because the former can work in extreme temperatures and deliver lots of current over a short period.
Indeed, a phenomenon related to discharge speed is what prompted the Golden Valley Electric Association (GVEA; Fairbanks, Alaska) to choose nickel-cadmium units to power its Battery Energy Storage System (BESS). Commissioned in late 2003, the GVEA system (Figure 3) is the biggest of its kind in the world. With the internal NiCad units connected in series to create a 5,000-VDC battery, BESS is capable of providing 27 MW of AC current for 15 minutes, or up to 46 MW for long enough to start up a backup generator to replace one that fails. Tim Devries, GVEA’s manager of engineering services, expects about 30 of these events per year in Alaska’s unforgiving climate.

3. Big BESS. At the heart of the Golden Valley Electric Association’s Battery Energy Storage System are racks holding 13,760 nickel-cadmium modules. Connected in series, the modules function as a 5,000-VDC battery.
Courtesy: Golden Valley Electric Association
GVEA chose NiCad batteries to power BESS (which happens to be in a controlled-temperature facility) because their capacity degrades slowly and linearly when they are discharged rapidly. During the selection process, Devries realized that meant he wouldn’t have to replace the NiCads at all during the system’s 20-year expected life. Over that period, had he chosen lead-acid batteries to power BESS, they would have to be replaced at least once. Add the capital and shipping costs of the new batteries to the cost of sending the old ones down to the Lower 48 for recycling, and you can see why the NiCads should end up costing less over two decades. Even better, Jim McDowall of Saft America Inc. (North Haven, Conn.), which provided the NiCads, expects that GVEA will be able to use BESS for more than 20 years, but at a lower output.
Perhaps vanadium redox
The two major suppliers of vanadium redox batteries are Tokyo-based Sumitomo Electric Industries Ltd. (SEI) and the aforementioned VRB Power Systems Inc. (VPS). Both companies rely on intellectual property held by Sydney-based Pinnacle VRB Ltd., in which VPS owns a controlling share. In addition, a third company—Reliable Power Inc. (Arlington, Va.)—is authorized by Sumitomo to sell vanadium redox batteries in the U.S.
SEI has been developing vanadium redox batteries since 1985 and manufacturing and marketing them since 2001. SEI markets megawatt-scale systems directly in Japan, where 11 projects representing more than 3.7 MW and 13.8 MWh are in operation.
To date, VPS has installed only two systems. One is a 200-kW/1,000-kWh battery connected to a hybrid wind/diesel-powered microgrid on King Island off the south coast of Australia. The other is a 350-kVA/2.4-MWh system (Figure 4) that provides peak shaving and voltage support for a 200-mile-long, 25-kV feeder on the PacifiCorp grid near Moab, Utah; it began operation in March 2004 (POWER, July/August 2004, p. 8). According to VPS President Vince Sorace, "Since the project has been up and running, feeder deviations have improved by 2% and the power factor improvement has reduced line losses by 40 kW."

4. On the job. VRB Power Systems dedicated this 350-kVA/2.4-MWh vanadium redox battery near Moab, Utah, in March 2004. It provides peak shaving and voltage support on a 200-mile-long PacifiCorp feeder.
Courtesy: VRB Power Systems Inc.
Consider zinc-bromide
Currently, ZBB Energy Corp. is the only company working to commercialize the zinc-bromide flow battery. The firm has its headquarters and a new production facility in Menominee Falls, Wis., but its research, development, and marketing efforts are based in Perth, Australia. Greg Nelson, the company’s chief operating officer, characterizes the company’s efforts as being in the small-scale demonstration phase.
To date, ZBB has largely focused on demonstrating the technology in the utility market. The company has built energy storage systems whose outputs range from 50 to 500 kWh for PG&E, Melbourne-based United Energy Ltd., Detroit Edison Co., Osaka-based Daihen Corp., and Sandia National Laboratories.
In the new factory, ZBB Energy soon will be able to build up to 100 MWh of battery systems per year—five times its current production limit. A commercially available 500-kWh grid-interactive storage system is the company’s basic building-block product. One system should satisfy most industrial energy storage needs, and several can be combined into multi-megawatt-hour sizes for utility applications (Figure 5). For example, four of the trailer-mounted systems are scheduled to be installed this year to provide 2 MW of peak-shaving support to a stressed PG&E substation.

5. One-stop shopping for moving and storage. This turnkey zinc-bromide flow battery from ZBB Energy Corp. can supply 250 kW for up to two hours. Delivered inside a standard shipping container, it is trailer-mounted, making it relocatable.
Courtesy: ZBB Energy Corp.
—Dan Greenberg, Ira Krepchin, and Kristin Kamm of the Platts E SOURCE Technology Assessment Group assisted in the preparation of this article.